Antibody data
- Antibody Data
- Antigen structure
- References [84]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [2]
- Other assay [80]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 71-1400 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- ZO-2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Aeromonas sobria Serine Protease Degrades Several Protein Components of Tight Junctions and Assists Bacterial Translocation Across the T84 Monolayer.
Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression.
The blood-brain barrier studied in vitro across species.
Zonula occludens 2 and Cell-Cell Contacts Are Required for Normal Nuclear Shape in Epithelia.
CCR5 antagonist reduces HIV-induced amyloidogenesis, tau pathology, neurodegeneration, and blood-brain barrier alterations in HIV-infected hu-PBL-NSG mice.
Tight junction protein ZO-2 modulates the nuclear accumulation of transcription factor TEAD.
The Na+, K+-ATPase β1 subunit regulates epithelial tight junctions via MRCKα.
Gut microbiota-CRAMP axis shapes intestinal barrier function and immune responses in dietary gluten-induced enteropathy.
ZO-1 Regulates Intercalated Disc Composition and Atrioventricular Node Conduction.
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity.
The homophilic receptor PTPRK selectively dephosphorylates multiple junctional regulators to promote cell-cell adhesion.
Japanese encephalitis virus neuropenetrance is driven by mast cell chymase.
E-cadherin is downregulated in benign prostatic hyperplasia and required for tight junction formation and permeability barrier in the prostatic epithelial cell monolayer.
Activation of the Ca(2+) sensing receptor and the PKC/WNK4 downstream signaling cascade induces incorporation of ZO-2 to tight junctions and its separation from 14-3-3.
HPV16-E6 Oncoprotein Activates TGF-β and Wnt/β-Catenin Pathways in the Epithelium-Mesenchymal Transition of Cataracts in a Transgenic Mouse Model.
ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner.
TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice.
Sorting nexin 27 interactome in T-lymphocytes identifies zona occludens-2 dynamic redistribution at the immune synapse.
ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway.
Heterogeneity between triple negative breast cancer cells due to differential activation of Wnt and PI3K/AKT pathways.
Homoharringtonine increases intestinal epithelial permeability by modulating specific claudin isoforms in Caco-2 cell monolayers.
Developmentally dynamic colocalization patterns of DSCAM with adhesion and synaptic proteins in the mouse retina.
Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion.
The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions.
p38δ mitogen-activated protein kinase regulates the expression of tight junction protein ZO-1 in differentiating human epidermal keratinocytes.
ZO proteins redundantly regulate the transcription factor DbpA/ZONAB.
Papillomavirus E6 oncoprotein up-regulates occludin and ZO-2 expression in ovariectomized mice epidermis.
12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction.
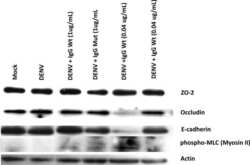
The cytokine response of U937-derived macrophages infected through antibody-dependent enhancement of dengue virus disrupts cell apical-junction complexes and increases vascular permeability.
Zona occludens-2 protects against podocyte dysfunction induced by ADR in mice.
The intracellular fate of zonula occludens 2 is regulated by the phosphorylation of SR repeats and the phosphorylation/O-GlcNAcylation of S257.
Unique cell type-specific junctional complexes in vascular endothelium of human and rat liver sinusoids.
ZO-1 regulates Erk, Smad1/5/8, Smad2, and RhoA activities to modulate self-renewal and differentiation of mouse embryonic stem cells.
Role of tight junction proteins in gastroesophageal reflux disease.
Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells.
Foxa2 mediates critical functions of prechordal plate in patterning and morphogenesis and is cell autonomously required for early ventral endoderm morphogenesis.
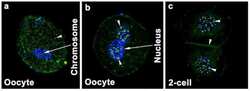
Dynamics of zonula occludens-2 expression during preimplantation embryonic development in the hamster.
Tight junctions in brain barriers during central nervous system inflammation.
Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis.
A role for ZO-1 and PLEKHA7 in recruiting paracingulin to tight and adherens junctions of epithelial cells.
Ouabain modulates ciliogenesis in epithelial cells.
Effects of long-term progesterone on developmental and functional aspects of porcine uterine epithelia and vasculature: progesterone alone does not support development of uterine glands comparable to that of pregnancy.
Identification of ZASP, a novel protein associated to Zona occludens-2.
Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep.
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Phosphorylation of zona occludens-2 by protein kinase C epsilon regulates its nuclear exportation.
Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle.
An immunochemical marker for goldfish Mauthner cells.
Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse.
Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression.
Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node.
Rab4A GTPase catenin interactions are involved in cell junction dynamics in the testis.
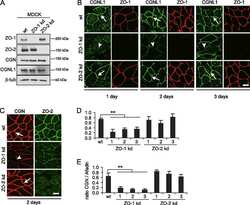
ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture.
Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc.
Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone.
Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina.
Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes.
Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells.
Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells.
Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner.
Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression.
Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells.
The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells.
The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells.
Functional and structural alterations of epithelial barrier properties of rat ileum following X-irradiation.
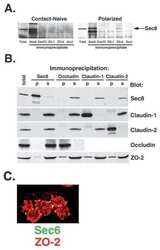
Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells.
Histone deacetylase inhibitors up-regulate the expression of tight junction proteins.
The specific fates of tight junction proteins in apoptotic epithelial cells.
The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B.
Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation.
Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins.
Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A.
Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions.
Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells.
Retention of the Alzheimer's amyloid precursor fragment C99 in the endoplasmic reticulum prevents formation of amyloid beta-peptide.
Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling.
Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling.
The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction.
Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules.
Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin.
Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin.
Ueda M, Kobayashi H, Seike S, Takahashi E, Okamoto K, Yamanaka H
Frontiers in cellular and infection microbiology 2022;12:824547
Frontiers in cellular and infection microbiology 2022;12:824547
Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression.
Delgado-Diaz DJ, Jesaveluk B, Hayward JA, Tyssen D, Alisoltani A, Potgieter M, Bell L, Ross E, Iranzadeh A, Allali I, Dabee S, Barnabas S, Gamieldien H, Blackburn JM, Mulder N, Smith SB, Edwards VL, Burgener AD, Bekker LG, Ravel J, Passmore JS, Masson L, Hearps AC, Tachedjian G
Microbiome 2022 Aug 31;10(1):141
Microbiome 2022 Aug 31;10(1):141
The blood-brain barrier studied in vitro across species.
Thomsen MS, Humle N, Hede E, Moos T, Burkhart A, Thomsen LB
PloS one 2021;16(3):e0236770
PloS one 2021;16(3):e0236770
Zonula occludens 2 and Cell-Cell Contacts Are Required for Normal Nuclear Shape in Epithelia.
Hernández-Guzmán C, Gallego-Gutiérrez H, Chávez-Munguía B, Martín-Tapia D, González-Mariscal L
Cells 2021 Sep 28;10(10)
Cells 2021 Sep 28;10(10)
CCR5 antagonist reduces HIV-induced amyloidogenesis, tau pathology, neurodegeneration, and blood-brain barrier alterations in HIV-infected hu-PBL-NSG mice.
Bhargavan B, Woollard SM, McMillan JE, Kanmogne GD
Molecular neurodegeneration 2021 Nov 22;16(1):78
Molecular neurodegeneration 2021 Nov 22;16(1):78
Tight junction protein ZO-2 modulates the nuclear accumulation of transcription factor TEAD.
Gallego-Gutiérrez H, González-González L, Ramírez-Martínez L, López-Bayghen E, González-Mariscal L
Molecular biology of the cell 2021 Jul 15;32(15):1347-1358
Molecular biology of the cell 2021 Jul 15;32(15):1347-1358
The Na+, K+-ATPase β1 subunit regulates epithelial tight junctions via MRCKα.
Bai H, Zhou R, Barravecchia M, Norman R, Friedman A, Yu D, Lin X, Young JL, Dean DA
JCI insight 2021 Feb 22;6(4)
JCI insight 2021 Feb 22;6(4)
Gut microbiota-CRAMP axis shapes intestinal barrier function and immune responses in dietary gluten-induced enteropathy.
Ren Z, Pan LL, Huang Y, Chen H, Liu Y, Liu H, Tu X, Liu Y, Li B, Dong X, Pan X, Li H, Fu YV, Agerberth B, Diana J, Sun J
EMBO molecular medicine 2021 Aug 9;13(8):e14059
EMBO molecular medicine 2021 Aug 9;13(8):e14059
ZO-1 Regulates Intercalated Disc Composition and Atrioventricular Node Conduction.
Dai W, Nadadur RD, Brennan JA, Smith HL, Shen KM, Gadek M, Laforest B, Wang M, Gemel J, Li Y, Zhang J, Ziman BD, Yan J, Ai X, Beyer EC, Lakata EG, Kasthuri N, Efimov IR, Broman MT, Moskowitz IP, Shen L, Weber CR
Circulation research 2020 Jul 3;127(2):e28-e43
Circulation research 2020 Jul 3;127(2):e28-e43
Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability.
Miranda J, Martín-Tapia D, Valdespino-Vázquez Y, Alarcón L, Espejel-Nuñez A, Guzmán-Huerta M, Muñoz-Medina JE, Shibayama M, Chávez-Munguía B, Estrada-Gutiérrez G, Lievano S, Ludert JE, González-Mariscal L
Cells 2019 Sep 29;8(10)
Cells 2019 Sep 29;8(10)
Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity.
García-Aguirre I, Alamillo-Iniesta A, Rodríguez-Pérez R, Vélez-Aguilera G, Amaro-Encarnación E, Jiménez-Gutiérrez E, Vásquez-Limeta A, Samuel Laredo-Cisneros M, Morales-Lázaro SL, Tiburcio-Félix R, Ortega A, Magaña JJ, Winder SJ, Cisneros B
Aging cell 2019 Oct;18(5):e13002
Aging cell 2019 Oct;18(5):e13002
The homophilic receptor PTPRK selectively dephosphorylates multiple junctional regulators to promote cell-cell adhesion.
Fearnley GW, Young KA, Edgar JR, Antrobus R, Hay IM, Liang WC, Martinez-Martin N, Lin W, Deane JE, Sharpe HJ
eLife 2019 Mar 29;8
eLife 2019 Mar 29;8
Japanese encephalitis virus neuropenetrance is driven by mast cell chymase.
Hsieh JT, Rathore APS, Soundarajan G, St John AL
Nature communications 2019 Feb 11;10(1):706
Nature communications 2019 Feb 11;10(1):706
E-cadherin is downregulated in benign prostatic hyperplasia and required for tight junction formation and permeability barrier in the prostatic epithelial cell monolayer.
Li F, Pascal LE, Stolz DB, Wang K, Zhou Y, Chen W, Xu Y, Chen Y, Dhir R, Parwani AV, Nelson JB, DeFranco DB, Yoshimura N, Balasubramani GK, Gingrich JR, Maranchie JK, Jacobs BL, Davies BJ, Hrebinko RL, Bigley JD, McBride D, Guo P, He D, Wang Z
The Prostate 2019 Aug;79(11):1226-1237
The Prostate 2019 Aug;79(11):1226-1237
Activation of the Ca(2+) sensing receptor and the PKC/WNK4 downstream signaling cascade induces incorporation of ZO-2 to tight junctions and its separation from 14-3-3.
Amaya E, Alarcón L, Martín-Tapia D, Cuellar-Pérez F, Cano-Cortina M, Ortega-Olvera JM, Cisneros B, Rodriguez AJ, Gamba G, González-Mariscal L
Molecular biology of the cell 2019 Aug 15;30(18):2377-2398
Molecular biology of the cell 2019 Aug 15;30(18):2377-2398
HPV16-E6 Oncoprotein Activates TGF-β and Wnt/β-Catenin Pathways in the Epithelium-Mesenchymal Transition of Cataracts in a Transgenic Mouse Model.
Rodríguez-Uribe G, Serafín-Higuera N, Damian-Morales G, Cortés-Malagón EM, García-Hernández V, Verdejo-Torres O, Campos-Blázquez JP, Trejo-Muñoz CR, Contreras RG, Ocadiz-Delgado R, Palacios-Reyes C, Lambert PF, Griep AE, Mancilla-Percino T, Escobar-Herrera J, Álvarez-Ríos E, Ugarte-Briones C, Moreno J, Gariglio P, Bonilla-Delgado J
BioMed research international 2018;2018:2847873
BioMed research international 2018;2018:2847873
ZO-1 protein is required for hydrogen peroxide to increase MDCK cell paracellular permeability in an ERK 1/2-dependent manner.
Bilal S, Jaggi S, Janosevic D, Shah N, Teymour S, Voronina A, Watari J, Axis J, Amsler K
American journal of physiology. Cell physiology 2018 Sep 1;315(3):C422-C431
American journal of physiology. Cell physiology 2018 Sep 1;315(3):C422-C431
TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice.
Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E
Brain, behavior, and immunity 2018 Mar;69:556-567
Brain, behavior, and immunity 2018 Mar;69:556-567
Sorting nexin 27 interactome in T-lymphocytes identifies zona occludens-2 dynamic redistribution at the immune synapse.
Tello-Lafoz M, Martínez-Martínez G, Rodríguez-Rodríguez C, Albar JP, Huse M, Gharbi S, Merida I
Traffic (Copenhagen, Denmark) 2017 Aug;18(8):491-504
Traffic (Copenhagen, Denmark) 2017 Aug;18(8):491-504
ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway.
Domínguez-Calderón A, Ávila-Flores A, Ponce A, López-Bayghen E, Calderón-Salinas JV, Luis Reyes J, Chávez-Munguía B, Segovia J, Angulo C, Ramírez L, Gallego-Gutiérrez H, Alarcón L, Martín-Tapia D, Bautista-García P, González-Mariscal L
Molecular biology of the cell 2016 May 15;27(10):1581-95
Molecular biology of the cell 2016 May 15;27(10):1581-95
Heterogeneity between triple negative breast cancer cells due to differential activation of Wnt and PI3K/AKT pathways.
Martínez-Revollar G, Garay E, Martin-Tapia D, Nava P, Huerta M, Lopez-Bayghen E, Meraz-Cruz N, Segovia J, González-Mariscal L
Experimental cell research 2015 Nov 15;339(1):67-80
Experimental cell research 2015 Nov 15;339(1):67-80
Homoharringtonine increases intestinal epithelial permeability by modulating specific claudin isoforms in Caco-2 cell monolayers.
Watari A, Hashegawa M, Yagi K, Kondoh M
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2015 Jan;89:232-8
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2015 Jan;89:232-8
Developmentally dynamic colocalization patterns of DSCAM with adhesion and synaptic proteins in the mouse retina.
de Andrade GB, Kunzelman L, Merrill MM, Fuerst PG
Molecular vision 2014;20:1422-33
Molecular vision 2014;20:1422-33
Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion.
Qiao X, Roth I, Féraille E, Hasler U
Cell cycle (Georgetown, Tex.) 2014;13(19):3059-75
Cell cycle (Georgetown, Tex.) 2014;13(19):3059-75
The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions.
Steiner E, Enzmann GU, Lyck R, Lin S, Rüegg MA, Kröger S, Engelhardt B
Cell and tissue research 2014 Nov;358(2):465-79
Cell and tissue research 2014 Nov;358(2):465-79
p38δ mitogen-activated protein kinase regulates the expression of tight junction protein ZO-1 in differentiating human epidermal keratinocytes.
Siljamäki E, Raiko L, Toriseva M, Nissinen L, Näreoja T, Peltonen J, Kähäri VM, Peltonen S
Archives of dermatological research 2014 Mar;306(2):131-41
Archives of dermatological research 2014 Mar;306(2):131-41
ZO proteins redundantly regulate the transcription factor DbpA/ZONAB.
Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S
The Journal of biological chemistry 2014 Aug 8;289(32):22500-11
The Journal of biological chemistry 2014 Aug 8;289(32):22500-11
Papillomavirus E6 oncoprotein up-regulates occludin and ZO-2 expression in ovariectomized mice epidermis.
Hernández-Monge J, Garay E, Raya-Sandino A, Vargas-Sierra O, Díaz-Chávez J, Popoca-Cuaya M, Lambert PF, González-Mariscal L, Gariglio P
Experimental cell research 2013 Oct 15;319(17):2588-603
Experimental cell research 2013 Oct 15;319(17):2588-603
12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction.
Kundumani-Sridharan V, Dyukova E, Hansen DE 3rd, Rao GN
The Journal of biological chemistry 2013 May 31;288(22):15830-42
The Journal of biological chemistry 2013 May 31;288(22):15830-42
The cytokine response of U937-derived macrophages infected through antibody-dependent enhancement of dengue virus disrupts cell apical-junction complexes and increases vascular permeability.
Puerta-Guardo H, Raya-Sandino A, González-Mariscal L, Rosales VH, Ayala-Dávila J, Chávez-Mungía B, Martínez-Fong D, Medina F, Ludert JE, del Angel RM
Journal of virology 2013 Jul;87(13):7486-501
Journal of virology 2013 Jul;87(13):7486-501
Zona occludens-2 protects against podocyte dysfunction induced by ADR in mice.
Bautista-García P, Reyes JL, Martín D, Namorado MC, Chavez-Munguía B, Soria-Castro E, Huber O, González-Mariscal L
American journal of physiology. Renal physiology 2013 Jan 1;304(1):F77-87
American journal of physiology. Renal physiology 2013 Jan 1;304(1):F77-87
The intracellular fate of zonula occludens 2 is regulated by the phosphorylation of SR repeats and the phosphorylation/O-GlcNAcylation of S257.
Quiros M, Alarcón L, Ponce A, Giannakouros T, González-Mariscal L
Molecular biology of the cell 2013 Aug;24(16):2528-43
Molecular biology of the cell 2013 Aug;24(16):2528-43
Unique cell type-specific junctional complexes in vascular endothelium of human and rat liver sinusoids.
Géraud C, Evdokimov K, Straub BK, Peitsch WK, Demory A, Dörflinger Y, Schledzewski K, Schmieder A, Schemmer P, Augustin HG, Schirmacher P, Goerdt S
PloS one 2012;7(4):e34206
PloS one 2012;7(4):e34206
ZO-1 regulates Erk, Smad1/5/8, Smad2, and RhoA activities to modulate self-renewal and differentiation of mouse embryonic stem cells.
Xu J, Lim SB, Ng MY, Ali SM, Kausalya JP, Limviphuvadh V, Maurer-Stroh S, Hunziker W
Stem cells (Dayton, Ohio) 2012 Sep;30(9):1885-900
Stem cells (Dayton, Ohio) 2012 Sep;30(9):1885-900
Role of tight junction proteins in gastroesophageal reflux disease.
Mönkemüller K, Wex T, Kuester D, Fry LC, Kandulski A, Kropf S, Roessner A, Malfertheiner P
BMC gastroenterology 2012 Sep 20;12:128
BMC gastroenterology 2012 Sep 20;12:128
Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells.
Noda S, Tanabe S, Suzuki T
Journal of agricultural and food chemistry 2012 May 9;60(18):4628-33
Journal of agricultural and food chemistry 2012 May 9;60(18):4628-33
Foxa2 mediates critical functions of prechordal plate in patterning and morphogenesis and is cell autonomously required for early ventral endoderm morphogenesis.
Harrelson Z, Kaestner KH, Evans SM
Biology open 2012 Mar 15;1(3):173-81
Biology open 2012 Mar 15;1(3):173-81
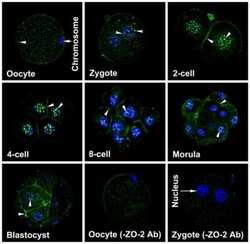
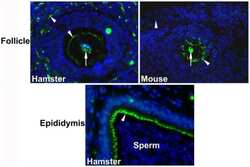
Dynamics of zonula occludens-2 expression during preimplantation embryonic development in the hamster.
Wang H, Luan L, Ding T, Brown N, Reese J, Paria BC
Theriogenology 2011 Sep 1;76(4):678-86
Theriogenology 2011 Sep 1;76(4):678-86
Tight junctions in brain barriers during central nervous system inflammation.
Coisne C, Engelhardt B
Antioxidants & redox signaling 2011 Sep 1;15(5):1285-303
Antioxidants & redox signaling 2011 Sep 1;15(5):1285-303
Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis.
Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B
Acta neuropathologica 2011 Nov;122(5):601-14
Acta neuropathologica 2011 Nov;122(5):601-14
A role for ZO-1 and PLEKHA7 in recruiting paracingulin to tight and adherens junctions of epithelial cells.
Pulimeno P, Paschoud S, Citi S
The Journal of biological chemistry 2011 May 13;286(19):16743-50
The Journal of biological chemistry 2011 May 13;286(19):16743-50
Ouabain modulates ciliogenesis in epithelial cells.
Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Muñoz-Estrada J, Cereijido M
Proceedings of the National Academy of Sciences of the United States of America 2011 Dec 20;108(51):20591-6
Proceedings of the National Academy of Sciences of the United States of America 2011 Dec 20;108(51):20591-6
Effects of long-term progesterone on developmental and functional aspects of porcine uterine epithelia and vasculature: progesterone alone does not support development of uterine glands comparable to that of pregnancy.
Bailey DW, Dunlap KA, Frank JW, Erikson DW, White BG, Bazer FW, Burghardt RC, Johnson GA
Reproduction (Cambridge, England) 2010 Oct;140(4):583-94
Reproduction (Cambridge, England) 2010 Oct;140(4):583-94
Identification of ZASP, a novel protein associated to Zona occludens-2.
Lechuga S, Alarcón L, Solano J, Huerta M, Lopez-Bayghen E, González-Mariscal L
Experimental cell research 2010 Nov 15;316(19):3124-39
Experimental cell research 2010 Nov 15;316(19):3124-39
Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep.
Sadowska GB, Malaeb SN, Stonestreet BS
American journal of physiology. Heart and circulatory physiology 2010 Jan;298(1):H179-88
American journal of physiology. Heart and circulatory physiology 2010 Jan;298(1):H179-88
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, Fukamachi H, Ito Y
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Phosphorylation of zona occludens-2 by protein kinase C epsilon regulates its nuclear exportation.
Chamorro D, Alarcón L, Ponce A, Tapia R, González-Aguilar H, Robles-Flores M, Mejía-Castillo T, Segovia J, Bandala Y, Juaristi E, González-Mariscal L
Molecular biology of the cell 2009 Sep;20(18):4120-9
Molecular biology of the cell 2009 Sep;20(18):4120-9
Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle.
Tapia R, Huerta M, Islas S, Avila-Flores A, Lopez-Bayghen E, Weiske J, Huber O, González-Mariscal L
Molecular biology of the cell 2009 Feb;20(3):1102-17
Molecular biology of the cell 2009 Feb;20(3):1102-17
An immunochemical marker for goldfish Mauthner cells.
Flores CE, Ene S, Pereda AE
Journal of neuroscience methods 2008 Oct 30;175(1):64-9
Journal of neuroscience methods 2008 Oct 30;175(1):64-9
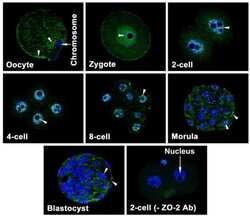
Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse.
Wang H, Ding T, Brown N, Yamamoto Y, Prince LS, Reese J, Paria BC
Developmental biology 2008 Jun 1;318(1):112-25
Developmental biology 2008 Jun 1;318(1):112-25
Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression.
Paschoud S, Citi S
Molecular membrane biology 2008 Jan;25(1):1-13
Molecular membrane biology 2008 Jan;25(1):1-13
Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node.
Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B
European journal of immunology 2008 Aug;38(8):2142-55
European journal of immunology 2008 Aug;38(8):2142-55
Rab4A GTPase catenin interactions are involved in cell junction dynamics in the testis.
Mruk DD, Lau AS, Sarkar O, Xia W
Journal of andrology 2007 Sep-Oct;28(5):742-54
Journal of andrology 2007 Sep-Oct;28(5):742-54
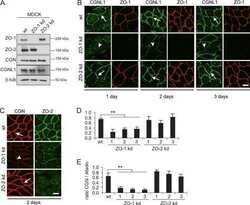
ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture.
Hernandez S, Chavez Munguia B, Gonzalez-Mariscal L
Experimental cell research 2007 May 1;313(8):1533-47
Experimental cell research 2007 May 1;313(8):1533-47
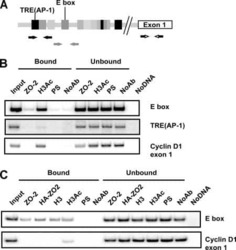
Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc.
Huerta M, Muñoz R, Tapia R, Soto-Reyes E, Ramírez L, Recillas-Targa F, González-Mariscal L, López-Bayghen E
Molecular biology of the cell 2007 Dec;18(12):4826-36
Molecular biology of the cell 2007 Dec;18(12):4826-36
Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone.
Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, Bazer FW
Endocrinology 2007 Aug;148(8):3922-31
Endocrinology 2007 Aug;148(8):3922-31
Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina.
Ciolofan C, Li XB, Olson C, Kamasawa N, Gebhardt BR, Yasumura T, Morita M, Rash JE, Nagy JI
Neuroscience 2006 Jun 30;140(2):433-51
Neuroscience 2006 Jun 30;140(2):433-51
Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes.
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW
The Journal of investigative dermatology 2005 Oct;125(4):761-74
The Journal of investigative dermatology 2005 Oct;125(4):761-74
Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells.
Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, Marasa BS, Wang JY
American journal of physiology. Gastrointestinal and liver physiology 2005 Jun;288(6):G1159-69
American journal of physiology. Gastrointestinal and liver physiology 2005 Jun;288(6):G1159-69
Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells.
Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE
American journal of physiology. Cell physiology 2005 Jun;288(6):C1231-41
American journal of physiology. Cell physiology 2005 Jun;288(6):C1231-41
Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner.
Singh D, Solan JL, Taffet SM, Javier R, Lampe PD
The Journal of biological chemistry 2005 Aug 26;280(34):30416-21
The Journal of biological chemistry 2005 Aug 26;280(34):30416-21
Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression.
Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S
Journal of cell science 2004 Oct 15;117(Pt 22):5245-56
Journal of cell science 2004 Oct 15;117(Pt 22):5245-56
Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells.
Jaramillo BE, Ponce A, Moreno J, Betanzos A, Huerta M, Lopez-Bayghen E, Gonzalez-Mariscal L
Experimental cell research 2004 Jul 1;297(1):247-58
Experimental cell research 2004 Jul 1;297(1):247-58
The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells.
Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, González-Mariscal L
Experimental cell research 2004 Jan 1;292(1):51-66
Experimental cell research 2004 Jan 1;292(1):51-66
The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells.
Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, González-Mariscal L
Experimental cell research 2004 Jan 1;292(1):51-66
Experimental cell research 2004 Jan 1;292(1):51-66
Functional and structural alterations of epithelial barrier properties of rat ileum following X-irradiation.
Dublineau I, Lebrun F, Grison S, Griffiths NM
Canadian journal of physiology and pharmacology 2004 Feb;82(2):84-93
Canadian journal of physiology and pharmacology 2004 Feb;82(2):84-93
Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells.
Yeaman C, Grindstaff KK, Nelson WJ
Journal of cell science 2004 Feb 1;117(Pt 4):559-70
Journal of cell science 2004 Feb 1;117(Pt 4):559-70
Histone deacetylase inhibitors up-regulate the expression of tight junction proteins.
Bordin M, D'Atri F, Guillemot L, Citi S
Molecular cancer research : MCR 2004 Dec;2(12):692-701
Molecular cancer research : MCR 2004 Dec;2(12):692-701
The specific fates of tight junction proteins in apoptotic epithelial cells.
Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O
Journal of cell science 2004 Apr 15;117(Pt 10):2097-107
Journal of cell science 2004 Apr 15;117(Pt 10):2097-107
The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B.
Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H
The Journal of biological chemistry 2003 Jan 24;278(4):2692-700
The Journal of biological chemistry 2003 Jan 24;278(4):2692-700
Isolation and characterization of human esophageal microvascular endothelial cells: mechanisms of inflammatory activation.
Rafiee P, Ogawa H, Heidemann J, Li MS, Aslam M, Lamirand TH, Fisher PJ, Graewin SJ, Dwinell MB, Johnson CP, Shaker R, Binion DG
American journal of physiology. Gastrointestinal and liver physiology 2003 Dec;285(6):G1277-92
American journal of physiology. Gastrointestinal and liver physiology 2003 Dec;285(6):G1277-92
Bryostatin-1 enhances barrier function in T84 epithelia through PKC-dependent regulation of tight junction proteins.
Yoo J, Nichols A, Mammen J, Calvo I, Song JC, Worrell RT, Matlin K, Matthews JB
American journal of physiology. Cell physiology 2003 Aug;285(2):C300-9
American journal of physiology. Cell physiology 2003 Aug;285(2):C300-9
Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A.
Chen ML, Pothoulakis C, LaMont JT
The Journal of biological chemistry 2002 Feb 8;277(6):4247-54
The Journal of biological chemistry 2002 Feb 8;277(6):4247-54
Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions.
Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC
The Journal of biological chemistry 2001 Sep 21;276(38):35571-80
The Journal of biological chemistry 2001 Sep 21;276(38):35571-80
Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells.
Yeaman C, Grindstaff KK, Wright JR, Nelson WJ
The Journal of cell biology 2001 Nov 12;155(4):593-604
The Journal of cell biology 2001 Nov 12;155(4):593-604
Retention of the Alzheimer's amyloid precursor fragment C99 in the endoplasmic reticulum prevents formation of amyloid beta-peptide.
Maltese WA, Wilson S, Tan Y, Suomensaari S, Sinha S, Barbour R, McConlogue L
The Journal of biological chemistry 2001 Jun 8;276(23):20267-79
The Journal of biological chemistry 2001 Jun 8;276(23):20267-79
Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling.
Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A
The Journal of cell biology 2001 Jul 23;154(2):369-87
The Journal of cell biology 2001 Jul 23;154(2):369-87
Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling.
Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A
The Journal of cell biology 2001 Jul 23;154(2):369-87
The Journal of cell biology 2001 Jul 23;154(2):369-87
The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction.
Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ
The Journal of biological chemistry 2000 Sep 22;275(38):29816-22
The Journal of biological chemistry 2000 Sep 22;275(38):29816-22
Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Ryeom SW, Paul D, Goodenough DA
Molecular biology of the cell 2000 May;11(5):1687-96
Molecular biology of the cell 2000 May;11(5):1687-96
Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Ryeom SW, Paul D, Goodenough DA
Molecular biology of the cell 2000 May;11(5):1687-96
Molecular biology of the cell 2000 May;11(5):1687-96
Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules.
Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL
Kidney international 2000 Jun;57(6):2386-402
Kidney international 2000 Jun;57(6):2386-402
Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin.
Cordenonsi M, D'Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S
The Journal of cell biology 1999 Dec 27;147(7):1569-82
The Journal of cell biology 1999 Dec 27;147(7):1569-82
Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin.
Cordenonsi M, D'Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S
The Journal of cell biology 1999 Dec 27;147(7):1569-82
The Journal of cell biology 1999 Dec 27;147(7):1569-82
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
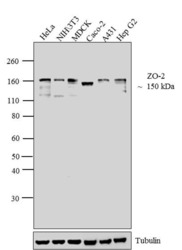
- Experimental details
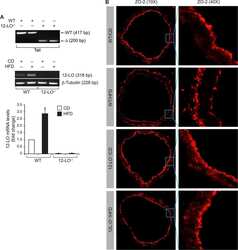
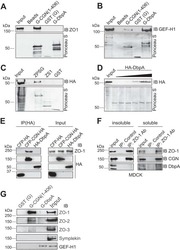
- Western blot analysis of ZO-2 was performed by loading 30 µg of HeLa (lane1), NIH\3T3 (lane2), MDCK (lane3), Caco-2 (lane4), A431 (lane5) and Hep G2 (lane6) cell lysate using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0322BOX), XCell SureLock™ Electrophoresis System (Product # EI0002), Novex® Sharp Pre-Stained Protein Standard (LC5800), and Pierce™ Power Blotter System (22834). Proteins were transferred to a nitrocellulose membrane and blocked with 5 % skim milk for 1 hour at room temperature. ZO-2 was detected at ~150 kDa using ZO-2 Rabbit Polyclonal Antibody (Product # 71-1400) at 1-2 µg/mL in 5 % skim milk at 4°C overnight on a rocking platform. Goat Anti-Rabbit IgG - HRP Secondary Antibody (G21234) at 1:5000 dilution was used and chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
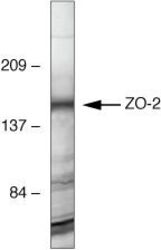
- Experimental details
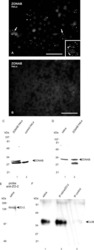
- Western blot analysis of MDCK cells using Rabbit anti-ZO-2 polyclonal antibody (Product # 71-1400).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
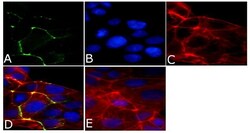
- Experimental details
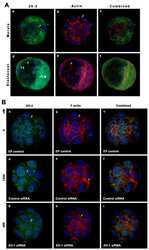
- Immunofluorescent analysis of ZO2/TJP2 Antibody was done on 90% confluent log phase CaCo2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with ZO2/TJP2 Antibody (Product # 71-1400) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing membrane localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
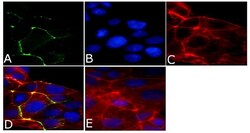
- Experimental details
- Immunofluorescent analysis of ZO2/TJP2 Antibody was done on 90% confluent log phase CaCo2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with ZO2/TJP2 Antibody (Product # 71-1400) at 1 µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing membrane localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
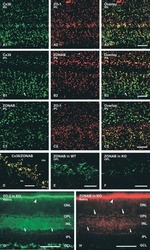
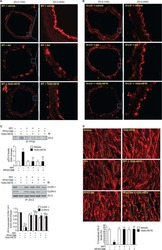
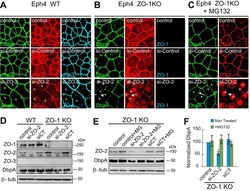
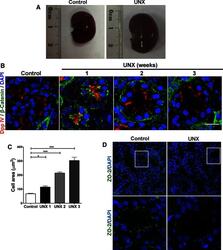
- FIGURE 6: Uninephrectomy triggered an increase in the size of the remaining kidney. (A) Images of kidneys from 11-wk-old rats. Left, kidney from a control animal; right, kidney from a rat in which the contralateral kidney had been removed 3 wk earlier. (B) Kidney frozen sections from 11-wk-old rats in control condition or that had experimented UNX 1-3 wk earlier. The apical brush border of proximal tubules is stained with specific antibodies against Dpp IV and the basolateral surface with anti-beta-catenin. (C) Quantification of the area of proximal tubule cells from kidneys of 11-wk-old rats (control) or that had experimented UNX 1-3 wk earlier. Area analysis was done using ImageJ on confocal images of frozen kidney sections stained with Dpp IV and anti-beta-catenin. A minimum of 53 cells was evaluated for each condition. Statistical analysis with one-way ANOVA, followed by Dunnett's test; * p < 0.05, *** p < 0.001. (D) ZO-2 expression is lost in the remaining kidneys of rats subjected to UNX. Frozen sections of kidneys from 11-wk-old rats that had been subjected or not to UNX 3 wk earlier were processed for immunofluorescence with an antibody against ZO-2, and the nuclei were stained with DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
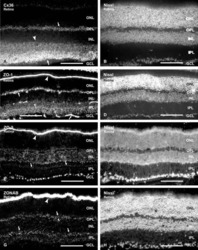
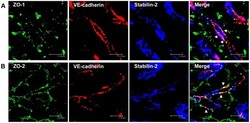
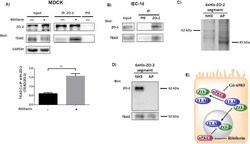
- Figure 5 ZO-1 and ZO-2 localize to VE-cadherin-containing cell-cell junctions in rat liver sinusoidal endothelial cells. (A, B) Immunofluorescent co-staining of rat liver cryosections with anti-ZO-1 (A, green), anti-ZO-2 (B, green), anti-VE-cadherin (A, B, red), and anti-Stabilin-2 (A, B, blue) antibodies. Images were acquired using laser scanning confocal microscopy. Bars 11.9 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
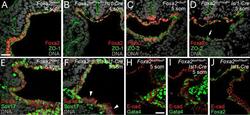
- Fig. 7. Decreased expression of proteins critical to ventral foregut morphogenesis in Foxa2loxP/loxP; Isl1-Cre embryos embryos. (A-D) Anti-Foxa2 and anti-ZO-1 (A-B) or anti-ZO-2 (C-D) coimmunohistochemistry (IHC) on Foxa2+/loxP (A, C) and Foxa2loxP/loxP; Isl1-Cre (B, D) embryos with 4-5 somite pairs (som). (A-B) Sections posterior to the anterior intestinal portal. (C-D) Sections through the foregut. White arrow in D marks a cluster of cells retaining expression of both Foxa2 and ZO-2. (E-F) Sections of anti-SOX17, anti-E-cadherin co-IHC near the anterior intestinal portal of Foxa2+/loxP (E) and Foxa2loxP/loxP; Isl1-Cre (F) embryos with 5 som. Segments of ventral foregut endoderm lacking Sox17 expression were observed in the mutant (white arrowheads). (G-H) Anti-Gata4, anti-E-cadherin (E-cad) co- IHC on sections of Foxa2loxP/loxP (G) and Foxa2loxP/loxP; Isl1-Cre (H) embryos with 5 somite pairs (som). Sections are posterior to the anterior intestinal portal. E-cadherin localizes to epithelial membranes and marks foregut endoderm. (I) Anti-Foxa2, anti-E-cadherin co-IHC on a section adjacent to that shown in B.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
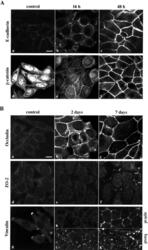
- Figure 2. Induction of epithelial markers by 1 alpha ,25(OH) 2 D 3 in SW480-ADH cells. (A) Analysis by immunofluorescence and confocal laser scanning microscopy of the expression of various adhesion proteins in cells treated with 10 -7 M 1alpha,25(OH) 2 D 3 for the indicated times or left untreated (control): E-cadherin (a-c); beta-catenin (d-f). (B) Same as in A with longer treatments: a-c, occludin; d-f, ZO-2; g-k, vinculin. Vinculin expression was analyzed at two sections: basal (g, i, and k) and apical (h and j). Bars, 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
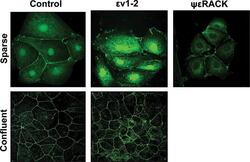
- Experimental details
- FIGURE 1: In confluent cultures, blocking the nuclear exportation of ZO-2 does not trigger the nuclear accumulation of the protein. MDCK cells were left untreated or incubated for 24 h with 1 muM PKCepsilon permeable inhibitor peptide epsilonv1-2 or PKCepsilon permeable activating peptide psiepsilonRACK. Monolayers were fixed, permeabilized, and processed for immunofluorescence with an antibody against ZO-2 (green). In control sparse cultures ZO-2 is present at the nucleus and cell borders, whereas in control confluent cultures, ZO-2 is absent from the nucleus and concentrates at the cell boundaries. Treatment with epsilonv1-2 conspicuously concentrates ZO-2 at the nucleus of sparse monolayers, whereas no change is observed in confluent cultures. Treatment of sparse monolayers with psiepsilonRACK depletes the nucleus of ZO-2.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
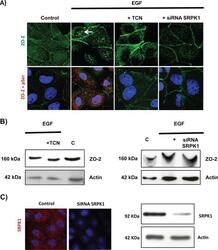
- Experimental details
- FIGURE 10: EGF induces, via Akt and with the participation of SRPK1, phosphorylation of ZO-2 in serine residues and triggers accumulation of the protein in nuclear speckles. Sparse cultures of MDCK cells transfected or not with a siRNA for SRPK1 were treated for 24 h with 100 nM EGF or EGF plus 30 nM TCN. (A) Top, treatment with EGF induces the appearance of ZO-2 in nuclear speckles (arrow) through a process sensitive to AKT inhibition with TCN and mediated by the presence of SRPK1. Cells were fixed and processed for immunofluorescence with antibodies against ZO-2. Bottom, EGF triggers increased phosphorylation of ZO-2 at serine residues through a process mediated by Akt and requiring the expression of SRPK1. The Duolink in situ immunoassay was done with an antibody against ZO-2 and an antibody against pSer. Cell borders were stained with an anti-E-cadherin antibody. (B) The shift in the ZO-2 band triggered by EGF in an SDS-PAGE with acrylamide-pendant Phos-tag disappeared upon incubation with TCN or by transfection of MDCK cells with a siRNA for SRPK1. (C) Transfection of a siRNA for SRPK1 decreases in MDCK cells the immunofluorescence (left, red) and Western blot (right) signals of the protein detected with a specific antibody against SRPK1. Nuclei were stained with DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
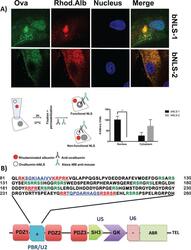
- Experimental details
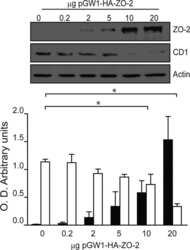
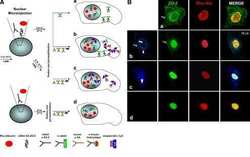
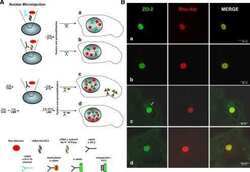
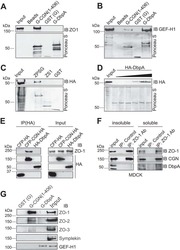
- FIGURE 2: ZO-2 sequence has two bNLSs, but only bNLS-1 is functional in a reporter protein nuclear importation assay. (A) Reporter protein nuclear microinjection assay. Bottom, left, schematic representation of the assay, illustrating how the cytoplasm of sparse MDCK cells was microinjected with the reporter protein ovalbumin chemically coupled to a peptide homologous to ZO-2 bNLSs (bNLS-1-OVA or bNLS-2-OVA) and rhodaminated albumin (Rho-Alb). After a 2-h incubation at 37degC, the cells were fixed and processed for immunofluorescence with an antibody against ovalbumin and a corresponding secondary antibody coupled to Alexa Fluor 488. Top, immunofluorescence obtained from the nuclear importation assay. Bottom right, quantitative analysis of ovalbumin fluorescence signal intensity in the nucleus and cytoplasm made with ImageJ (National Institutes of Health, Bethesda, MD) particle analysis. Results from three independent experiments. * p < 0.05 as assessed by two-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test. bNLS-1-OVA (green) concentrates at the nucleus stained in blue with TO-PRO-3 (blue), whereas rhodaminated albumin (red) distributes in the cytoplasm. bNLS-2-OVA does not travel to the nucleus and remains in the cytoplasm. (B) Schematic representation of ZO-2 showing a polybasic region (+) located at the amino-terminal segment of PDZ-1 and the beginning of the U2 linker between PDZ-1 and PDZ-2 domains. This region contains 16 RS motifs (green),
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
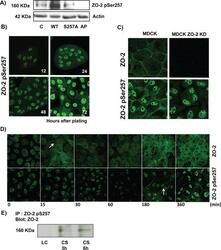
- Experimental details
- FIGURE 4: Phosphorylation of ZO-2 at S257 occurs in mature TJs. (A) Western blot with the antibody against ZO-2 S257-P in a lysate derived from control cells (C) untreated or after alkaline phosphatase treatment (AP) or in cells transfected with WT ZO-2 or ZO-2 mutant S257A. (B) ZO-2 S257-P is present at the cell borders at late but not at early times after plating. MDCK monolayers plated at subconfluent density were fixed at different times after plating and processed for immunofluorescence with a specific antibody against ZO-2 S257-P. (C) Nuclear staining with the antibody against ZO-2 S257-P is unspecific. An antibody against ZO-2 gives no staining in ZO-2 KD monolayers, whereas the ZO-2 S257-P antibody gives a nuclear labeling in ZO-2-KD cells. (D) Phosphorylation of ZO-2 at S257 occurs in mature TJs. ZO-2 appears at the cell borders 15 min after the Ca 2+ switch, whereas ZO-2 S257-P is detected 180 min after transfer to NC. (E) The amount of ZO-2 S257-P increases as cell-cell contacts mature in a Ca 2+ switch assay. ZO-2 S257-P was immunoprecipitated from monolayers incubated 20 h in low calcium or subsequently transferred to normal calcium for 3 and 6 h.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
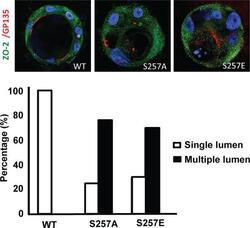
- FIGURE 5: S257 in ZO-2 is required for the correct formation of cysts. MDCK cells transfected with ZO-2 WT or the mutant S257A or S257E were incubated with Matrigel and observed 8 d later to allow the formation of cell cysts. Cysts were fixed and processed for immunofluorescence with antibodies against ZO-2 (green) and gp135/podocalyxin (red). The nuclei were stained in blue with DAPI dissolved in mounting medium. The images (top) and the graph with the quantitated data (bottom) show that the substitution of S257 with either alanine or glutamic acid induces the aberrant formation of multiple lumens per cyst. A total of 50 cysts were analyzed per experimental condition. In comparison to WT all conditions were statistically significant, p < 0.001, using a chi-squared test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
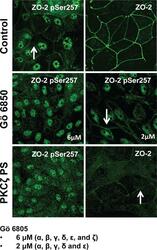
- FIGURE 6: PKCzeta phosphorylates ZO-2 at S257. MDCK cells were plated at confluence density in the absence or presence of 2 or 6 muM Go 6850 or 10 muM PKCzeta pseudosubstrate. One day later the monolayers were fixed and processed for immunofluorescence with antibodies against ZO-2 or ZO-2 S257-P. Observe the disappearance of the ""chicken-fence"" pattern of ZO-2 S257-P upon treatment with PKCzeta pseudosubstrate (PS) or 6 muM Go 6850.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
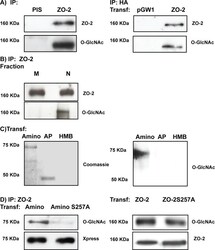
- FIGURE 7: ZO-2 is O -GlcNAcylated at the nucleus at S257. (A) ZO-2 is an O -GlcNAcylated protein. Endogenous (left) or exogenous (right) ZO-2 was immunoprecipitated with antibodies against ZO-2 (left) or HA (right) and blotted with antibodies against ZO-2, HA, or O -GlcNAc. PIS, preimmune serum; pGW1, empty vector. (B) O -GlcNAcylated ZO-2 is present in the nucleus but not in the plasma membrane. Endogenous ZO-2 was immunoprecipitated from a membrane (M) or nuclear (N) fraction derived from MDCK cells and blotted with an antibody against O -GlcNAc. (C) The amino and not the AP segment of ZO-2 is O -GlcNAcylated. MDCK cells were transfected with amino and AP histidine-tagged constructs of ZO-2. The corresponding proteins were purified by Ni affinity columns, run in SDS-PAGE, stained with Coomassie blue, and blotted with an antibody against O -GlcNAc. HMB, empty vector HisMax B. (D) ZO-2 is O -GlcNAcylated at S257 and elsewhere outside of the amino segment. MDCK cells were transfected with ZO-2 WT amino or amino 257A constructs (left) or ZO-2 full-length WT or mutant 257A (right). ZO-2 was immunoprecipitated, run on SDS-PAGE, and blotted against O -GlcNAc and the Xpress tag. Transf, transfection.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
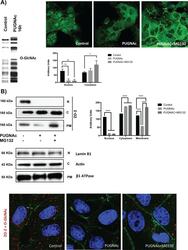
- FIGURE 8: PUGNAc-induced stabilization of O -GlcNAcylation promotes nuclear exportation and degradation of ZO-2. Sparse cultures of MDCK cells were treated or not for 16 h with 100 muM PUGNAc or 100 muM PUGNAc plus 30 mM MG132. (A) Western blot detection of O -GlcNAc in MDCK cell extracts treated or not with PUGNAc (left). PUGNAc induces the disappearance of ZO-2 from the nuclei and cellular borders. Monolayers were fixed and processed for immunofluorescence with an antibody against ZO-2. The quantitative analysis of ZO-2 fluorescence signal intensity in the nucleus and cytoplasm was made with ImageJ particle analysis (bottom right). Results from three independent experiments. * p < 0.05 and ** p < 0.01 as assessed by two-way ANOVA followed by Bonferroni's post hoc test. (B) Treatment with PUGNAc decreases nuclear ZO-2. Left, Western blot against ZO-2 of nuclear (N), cytoplasmic (C), and plasma membrane (PM) fractions derived from cells treated or not with PUGNAc or PUGNAc plus MG132. Antibodies against beta 1 -Na + -K + -ATPase, actin, and lamin B1 were used as positive controls of PM, C, and N fractions, respectively. Right, densitometric analysis of the Western blot. Results from three independent experiments. * p < 0.05, ** p < 0.01, and **** p < 0.0001 as assessed by two-way ANOVA followed by Bonferroni's post hoc test. (C) Treatment with PUGNAc ablates the expression of O -GlcNAcylated ZO-2. The Duolink in situ immunoassay was done with an antibody against ZO-
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
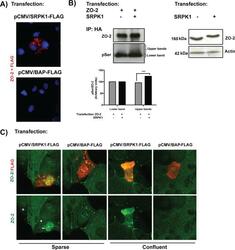
- FIGURE 9: SRPK1 phosphorylation of ZO-2 SR motifs induces the transport of the protein to the nucleus. (A) ZO-2 and SRPK1 associate in the cytoplasm. Duolink assay done with antibodies against ZO-2 and against FLAG in MDCK cells transfected with SRPK1-FLAG or the empty vector pCMV. Nuclei were stained in blue with DAPI. (B) Overexpression of SRPK1 induces serine hyperphosphorylation of ZO-2. Top left, MDCK cells were transfected with HA-ZO-2 or HA-ZO-2 plus SRPK1. Exogenous ZO-2 was immunoprecipitated with an anti-HA antibody and blotted with anti-pSer antibody, stripped, and blotted with anti-ZO-2. Bottom, densitometric analysis of the pSer/ZO-2 signal present in the upper and lower bands of the top left blot. In comparison to ZO-2 WT, ZO-2 WT/SRPK1 cotransfection was statistically significant, p < 0.001, using a chi-squared test. Top right, a total cellular extract of MDCK cells was transfected or not with SRPK1 and run on an SDS-PAGE with acrylamide-pendant Phos-tag. (C) Overexpression of SRPK1 induces the transport of ZO-2 into the nucleus. In sparse cells transfected with SRPK1, ZO-2 concentrates at the nucleus (arrow) and disappears from the cell borders (arrowhead).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
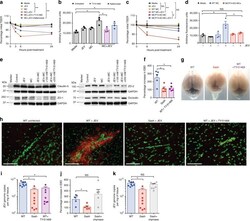
- Fig. 5 Inhibition of chymase abolishes JEV-induced breakdown of the BBB. a bEND.3 cell monolayers were treated with media alone or media containing JEV or supernatants of MCs only, JEV-stimulated MCs, or JEV-stimulated MCs treated with either TY-51469 (chymase inhibitor) or nafamostat mesylate (tryptase inhibitor). Supernatants from JEV-stimulated BMMCs reduced the TEER, which was reversed by TY-51469 (100 uM), but not nafamostat mesylate (10 uM); analyzed by two-way ANOVA. b JEV-activated BMMC supernatants increased FITC-dextran leakage across transwells 24 h post-exposure. TY-51469, but not nafamostat mesylate, reduced FITC-dextran leakage; n = 3. c Supernatants from JEV-stimulated WT BMMCs reduced TEER of b.END3 monolayers, but control media, JEV, supernatants from WT or MCPT4-KO BMMCs or JEV-stimulated MCPT4-KO BMMCs did not. d JEV-activated WT BMMC supernatants increased FITC-dextran leakage (24 h), but controls or supernatants from JEV-activated MCPT4-KO BMMCs did not; n = 3. e Claudin-5, ZO-1, ZO-2, and occludin levels in bEND.3 cells exposed to JEV-activated MC supernatants were reduced compared to media, JEV, and unstimulated MC supernatant-treated groups, by western blotting, which was inhibited by TY-51469. GAPDH blotting served as loading controls. Quantification is provided (Supplementary Figure 11 ). f TY-51469 reduced EBD leakage into brains of JEV-infected WT mice, to levels similar to JEV-infected Sash mice, 5 days post-i.p. infection with Nakayama (2 x 10 7
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
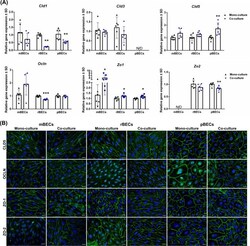
- 10.1371/journal.pone.0236770.g002 Fig 2 Expression of tight junction proteins. (A) Relative gene expression of claudin-1,-3,-5 ( Cld1 , Cld3 , and Cld5 ), occludin ( Ocln ), and zonula occludens 1 and 2 ( Zo1 , Zo2 ) in mouse, rat, and porcine brain capillary endothelial cells (mBECs, rBECs, pBECs) cultured as mono-culture (black triangle) and as co-culture with primary astrocytes (blue circle). The expression pattern of the different tight junction proteins is relatively similar across the three species. Co-culturing the BECs with astrocytes decreases the expression of Cld1 and Zo2 compared to mono-cultured BECs. Oppositely, a significant increase in the expression of Zo1 is seen after co-culturing the BECs. Cld3 , Cld5 , and Ocln are unaffected by the culturing conditions except when rBECs and pBECs are co-cultured with astrocytes, where a significantly lower expression of Ocln or a significant increase in Cld5 expression is seen, respectively. Despite multiple attempts, the expression of Cld3 and Zo2 is non-detectable in the pBECs and mBECs cultures, respectively. A change in gene expression between mono- and co-cultures for each species is analyzed using an unpaired t-test or non-parametric Mann-Whitney test, depending on the variance of the data. Data are shown as mean +- standard deviation (SD) (n = 6), * p < 0 . 05 , **p < 0 . 01 , ***p < 0 . 001 . (B) Immunofluorescent images showing green labeling at the cell-cell borders of CLD5, OLCN, ZO-1, and ZO-2 in mBECs, rBECs
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
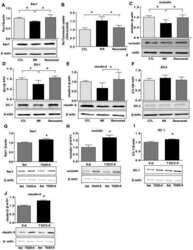
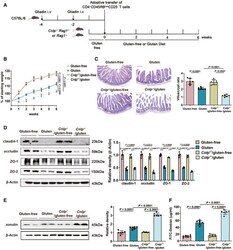
- Figure 2 CRAMP deficiency potentiates GIE Animal protocol. Changes in body weight relative to starting weight during 6 weeks ( n = 6). Left, representative images of duodenal damage by H&E staining. Scale bar: 200 mum. Right, graph depicted the ratio of the morphometric assessment of villus height to crypt depth ( n = 6). Western blot and densitometry analyses of duodenal tight junction proteins (claudin-1, occludin, ZO-1 and ZO-2; n = 6). Western blot and densitometry analysis of duodenal zonulin ( n = 6). Intestinal permeability was assessed by measuring FITC-Dextran ( n = 6). Data information: Data (B-F) were representative and were the mean +- SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
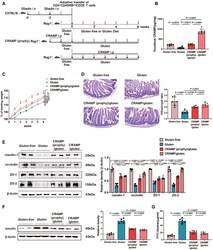
- Experimental details
- Figure 3 Replenishing duodenal CRAMP is protective against GIE Animal protocol. Duodenal CRAMP determination by ELISA ( n = 6). Changes in body weight relative to starting weight during 6 weeks ( n = 6). Left, representative images of duodenal damage by H&E staining. Scale bar: 200 mum. Right, graph depicted the ratio of the morphometric assessment of villus height to crypt depth ( n = 6). Western blot and densitometry analyses of duodenal tight junction proteins (claudin-1, occludin, ZO-1 and ZO-2; n = 6). Western blot and densitometry analysis of duodenal zonulin ( n = 6). Intestinal permeability was assessed by measuring FITC-Dextran ( n = 6). Data information: Data (B-G) were representative and were the mean +- SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
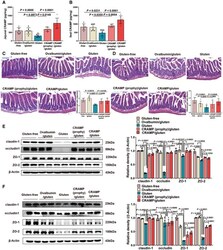
- Experimental details
- Figure EV2 Replenishing CRAMP is protective against jejunal and ileal barrier damage during GIE. Related to Fig 3 A, B CRAMP determinations in (A) jejunum and (B) ileum by ELISA ( n = 6). C, D Representative images of (C) jejunal and (D) ileal damage by H&E staining. Scale bar: 200 mum. The graphs depicted the ratio of the morphometric assessment of villus height to crypt depth ( n = 6). E, F Western blot and densitometry analyses of tight junction proteins (claudin-1, occludin, ZO-1 and ZO-2) in (E) jejunum and (F) ileum ( n = 4). Data information: Data were representative and were the mean +- SD from three (A-D) or two (E, F) independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. Source data are available online for this figure.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
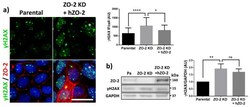
- Experimental details
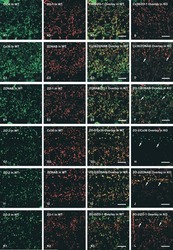
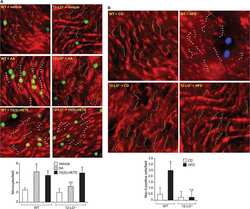
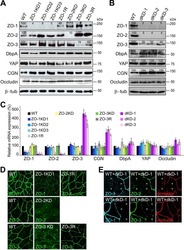
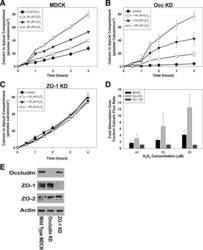
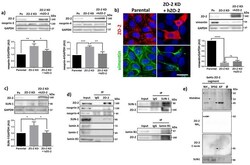
- Figure 2 U-H2AX nuclear foci are more abundant in ZO-2 KD than in parental MDCK cells. ( a ) Parental and ZO-2 KD MDCK cells transfected or not with hZO-2 were processed for immunofluorescence with an antibody against U-H2AX, a DDR marker. Cell borders were stained with antibodies against ZO-1, and the nuclei were detected with DAPI. Left, representative images, bar 20 mum; *, non-transfected cell. Right, quantitative analysis done with ImageJ. Data obtained from at least 38 cells derived from three optical fields in each experimental condition: parental cells, MDCK ZO-2 KD cells highly expressing transfected hZO-2, and ZO-2 KD cells without visible transfected hZO-2 signal. Statistical analysis was done with Kruskal-Wallis followed by Dunn's multiple comparisons test. Results are shown as media +- standard deviation. * p < 0.05, **** p < 0.0001. ( b ) Western blot detection of U-H2AX in Parental and ZO-2 KD MDCK cells transfected or not with hZO-2. GAPDH was employed as a loading control. Left panel, representative Western blot; right panel, quantitative analysis of three independent experiments. Statistical analysis was done with one-way ANOVA, followed by Tukey's multiple comparisons test. Results are shown as media +- standard deviation, ** p < 0.01; ns, non-significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
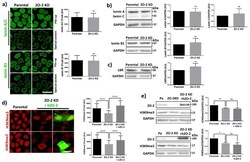
- Experimental details
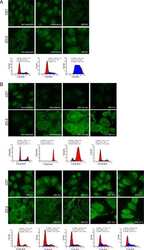
- Figure 4 In ZO-2 KD MDCK cells, lamins A/C and B1 and lamin B receptors are unaffected, while the expression of constitutive and facultative heterochromatin diminishes. ( a ) Monolayers of parental and ZO-2 KD MDCK cells were processed for immunofluorescence with antibodies against lamins A/C and B1. Left panels, representative images obtained of pile-ups or single sections; bar, 20 mum. Right, quantitative analysis was done with ImageJ of pile-up images. Data obtained with 200 nuclei in parental condition and 141 nuclei in ZO-2 KD condition for lamin A/C, and 380 nuclei per experimental condition for lamin B1, from two independent experiments. Statistical analysis was done with Mann-Whitney test. Results are shown as media +- standard deviation. ns, non-significant. ( b ) Western blot analysis of lamins A/C and B1 in parental and ZO-2 KD MDCK cells. GAPDH was employed as a loading control. Left, representative images; right quantitative analysis. Results from three independent experiments. Statistical analysis was done with Student's t -test. Results are shown as media +- standard deviation. ns, non-significant. ( c ) Western blot analysis of lamin B receptor (LBR) in parental and ZO-2 KD MDCK cells. GAPDH was employed as a loading control. Left, representative images; right quantitative analysis. Results from three independent experiments. Statistical analysis done with Student's t -test. Results are shown as media +- standard deviation. ns, non-significant. ( d ) Immunoflu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
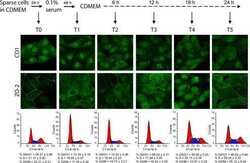
- Figure 6 The nuclear shape is altered when the cells are cultured in LC, lack ZO-2 or are devoid of cell-cell contacts. ( a ) Immunofluorescence of lamin B1, in sparse or confluent cultures of parental and ZO-2 KD cells, cultured for 24 h in low calcium (LC, 1-5 muM Ca 2+ ) or normal calcium (NC, 1.8 mM) media. Left panel, representative images; right panels, quantitative analysis of nuclear indentations. Statistical analysis was done with One-way ANOVA followed by Tukey's multiple comparisons test. Results are shown as media +- standard deviation. ns, non-significant; * p < 0.05; ** p = 0.01; *** p = 0.001; **** p < 0.0001. ( b ) Western blot analysis of ZO-2 present in cytoplasmic and nuclear fractions derived from sparse (S) and confluent (C) cultures of parental MDCK cells. Lamin B1 and GAPDH were employed as markers and loading controls for nuclear and cytoplasmic fractions, respectively. Left, representative images; right quantitative analysis. Results from three independent experiments. Statistical analysis was done with Student's t -test. Results are shown as media +- standard deviation. ns, non-significant; **** p < 0.0001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 7 ZO-2 associates to SUN-1 and lamin B1, and its absence triggers an increased expression of SUN-1 and nesprins-3, and -4 and reduces vimentin. ( a ) Western blot detection of nesprins-3 and -4 in parental and ZO-2 KD cells transfected or not with hZO-2. Upper panels, representative images; lower panels, quantitative analysis obtained from three independent experiments. GAPDH is used as a loading control. Statistical analysis was done with One-way ANOVA followed by Tukey's multiple comparisons test. Results shown as media +- standard deviation, * p < 0.05, ** p < 0.01, *** p < 0.001. ( b ) Left panel, representative immunofluorescence images showing ZO-2 and vimentin expression in parental and ZO-2 KD cells transfected or not with hZO-2. Bar, 20 mum. Right panel, representative Western blot of three independent experiments and corresponding quantitative analysis. GAPDH is used as a loading control. Statistical analysis was done with One-way ANOVA followed by Tukey's multiple comparisons test. Results are shown as media +- standard deviation, **** p < 0.0001; ns, non-significant. ( c ) Western blot detection of SUN-1 in parental and ZO-2 KD cells transfected or not with hZO-2. Upper panel, representative image; lower panel, quantitative analysis obtained from three independent experiments. GAPDH is used as a loading control. Statistical analysis was done with One-way ANOVA followed by Tukey's multiple comparisons test. Results are shown as media +- standard deviation, *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
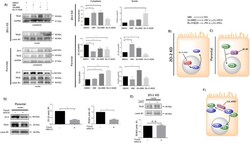
- Experimental details
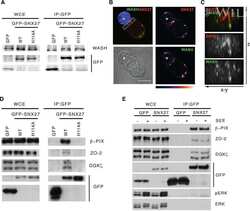
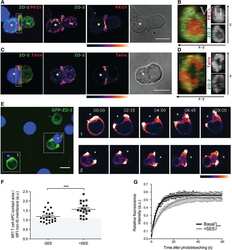
- FIGURE 3: ZO-2 modulates the effect exerted by PKC delta on TEAD nuclear accumulation. (A) Confluent monolayers of parental and ZO-2 KD MDCK cells were treated with 10 muM H89 to inhibit PKA, 27 nM Ro 31-8220 to inhibit cPKC alpha, gamma, and beta1 and nPKCepsilon, 10 nM Go-6986 to inhibit cPKC alpha, beta, and gamma and nPKCdelta, or 0.1% DMSO as vehicle. Cytoplasmic and nuclear fractions were isolated and run in a SDS-PAGE and blotted with antibodies against ZO-2 and TEAD. GAPDH and lamin B1 were employed as loading controls for cytoplasmic and nuclear fractions, respectively. Left, representative images of Western blots from three independent experiments; right, densitometric analysis. Statistics done with one-way ANOVA followed by Fisher's least significant difference test, * p < 0.05; ** p < 0.01; *** p < 0.001. Each bar shows the mean +- SEM. (B) Schematic representation showing that in ZO-2 KD MDCK cells Ro-31822 blocks the nuclear exportation of TEAD mediated by nPKCepsilon. (C) Schematic representation depicting in parental cells ZO-2 entry to the nucleus triggered by the inhibition of PKA with 10 muM H89. (D) Parental MDCK cells were transfected with a nPKCdelta construct, and 24 h later nuclear fractions were obtained and processed for Western blot employing antibodies against ZO-2 and TEAD. Lamin B1 was employed as loading control of nuclear fractions. Left, representative image of Western blots from three independent experiments; right, densitometric analysis. St
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 6: The interaction between TEAD and ZO-2 is inhibited by nPKCdelta. (A) The amount of TEAD that coimmunoprecipitates with ZO-2 is augmented in cells treated for 1 h with 3 muM rottlerin. Blotting was done with antibodies against ZO-2 and TEAD. Top panel, representative image; bottom panel, densitometric analysis. Statistics done with Students' t test, ** p < 0.01. Each bar shows the mean +- SEM. Co-IP, coimmunoprecipitation; IP, immunoprecipitation. (B) In extracts derived from intestinal IEC-18 cells, TEAD coimmunoprecipitates with ZO-2. PIS, preimmune serum. (C) 6XHis-tagged amino (NH3) and AP segments of cZO-2 were purified with Ni affinity columns from extracts of HEK293T cells, run in SDS-PAGE, and stained with Coomassie blue. (D) Western blot analysis of pull downs of amino and AP segments of cZO-2. Top panel, anti-ZO-2 antibody recognizes only the 3PSG segment of ZO-2. Bottom panel, anti-TEAD antibody gives a positive signal in pull downs of amino and AP segments of cZO-2. (E) Cartoon showing that the inhibition of nPKCdelta allows ZO-2-TEAD interaction, which facilitates the nuclear importation of these proteins. At the nucleus nPKCdelta also ruptures ZO-2-TEAD association.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
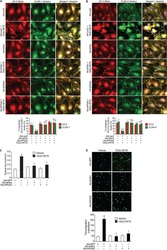
- Degradation of the protein components constituting TJs due to infection of various A. sobria strains to the apical side of the T84 intestinal epithelial monolayer. T84 cells were cultured in 12-well microplates and then infected with several A. sobria strains. After 2 hr of infection (MOI = 5) to the apical side of the T84 monolayer, the cell extracts were prepared. The protein components constituting TJs (ZO-1, ZO-2, ZO-3, occludin, and claudin-1, -3, -4, and -7) were detected by using a specific antibody against each protein. The results of the quantitative analysis of the relative ratio (%) of the amount of blotted protein (ZO-1, ZO-2, and ZO-3) to the amount of GAPDH are shown below the western blotting image. Concerning the degradation of claudin-7, the results of the quantitative analysis of the relative ratio (%) of the amount of the blotted claudin-7 degradation fragment (Claudin-7 FG) to the amount of GAPDH are shown. These experiments were performed in triplicate. The data are mean +- SD (error bars). We performed an ANOVA analysis with Dunnett's test and showed significant differences (*p < 0.05, **p < 0.01) between the data obtained from each bacterial strain and the control data (NT).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
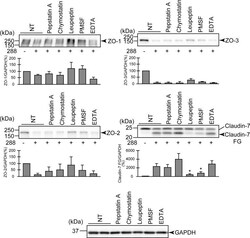
- Experimental details
- Effects of various protease inhibitors on the degradation of ZO-1, ZO-2, ZO-3, and claudin-7 due to infection of A. sobria strain 288 to the apical side of the T84 intestinal epithelial monolayer. T84 cells were cultured in 12-well microplates and then infected with A. sobria strain 288 in the presence of the protease inhibitors shown. After 2 hr of infection (MOI = 5) to the apical side of the T84 monolayer, the cell extracts were prepared. The protein components (ZO-1, ZO-2, ZO-3, and claudin-7) constituting TJs were detected by using a specific antibody against each protein. The results of the quantitative analysis of the relative ratio (%) of the amount of blotted protein (ZO-1, ZO-2, and ZO-3) to the amount of GAPDH are shown below the western blotting image. Concerning the degradation of claudin-7, the results of the quantitative analysis of the relative ratio (%) of the amount of the blotted Claudin-7 FG to the amount of GAPDH is shown. These experiments were performed in triplicate. The data are mean +- SD (error bars). *p < 0.05; significance was tested by comparison with the control data (NT; without infection with strain 288).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
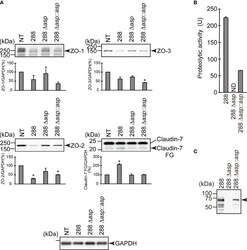
- Experimental details
- Effect of the asp gene disruption on the degradation of the protein components constituting TJs. (A) T84 cells were cultured in 12-well microplates and then infected with A. sobria strain 288 and its derivative strains shown in the figure. After 2 hr of infection (MOI = 5) to the apical side of the T84 monolayer, the cell extracts were prepared. The protein components (ZO-1, ZO-2, ZO-3, and claudin-7) constituting TJs were detected by using a specific antibody against each protein. The results of the quantitative analysis of the relative ratio (%) of the amount of blotted protein (ZO-1, ZO-2, and ZO-3) to the amount of GAPDH are shown below the western blotting image. Concerning the degradation of claudin-7, the results of the quantitative analysis of the relative ratio (%) of the amount of the blotted Claudin-7 FG to the amount of GAPDH are shown. These experiments were performed in triplicate. The data are mean +- SD (error bars). *p < 0.01; significance was tested by comparison with the control data (NT). (B) The proteolytic activity in the culture supernatant of each A. sobria strain was measured as described in the text. The experiments were performed in triplicate. ND: The proteolytic activity could not be sufficiently detected in this experimental condition. The data are mean +- SD (error bars). (C) The presence of ASP in the culture supernatant of each strain was immunologically detected by a western blotting analysis as described in the text. The arrow indicates the
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
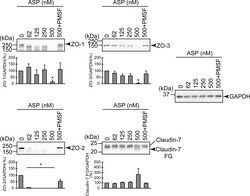
- Experimental details
- Effect of the purified ASP on various protein components constituting TJs. T84 cells were cultured in 12-well microplates and then treated with various concentrations (nM) of the purified ASP. For the evaluation of the effect of the serine protease inhibitor, T84 cells were also treated with 500 nM of the purified ASP in the presence of PMSF. After treatment for 2 hr, the cell extracts were prepared. The protein components (ZO-1, ZO-2, ZO-3, and claudin-7) constituting TJs were detected by using a specific antibody against each protein. The results of the quantitative analysis of the relative ratio (%) of the amount of blotted protein (ZO-1, ZO-2, and ZO-3) to the amount of GAPDH are shown below the western blotting image. Regarding the degradation of claudin-7, the results of the quantitative analysis of the relative ratio (%) of the amount of the blotted Claudin-7 FG to the amount of GAPDH are shown. These experiments were performed in triplicate. The data are mean +- SD (error bars). *p < 0.01; significance was tested by comparison with the control data (0 nM ASP).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
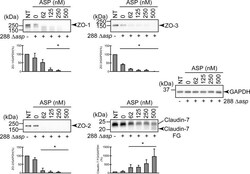
- Experimental details
- Degradation of ZO-1, ZO-2, ZO-3, and claudin-7 due to infection of the asp -deficient A. sobria strain (288 Delta asp ) to the apical side of the T84 intestinal epithelial monolayer in the presence of various concentrations of the purified ASP. T84 cells were cultured in 12-well microplates and then infected with the A. sobria 288 Delta asp strain in the presence of various concentrations of the purified ASP. After 2-hr treatment, the cell extracts were prepared. ZO-1, ZO-2, ZO-3, and claudin-7 were detected by using a specific antibody against each protein. The results of the quantitative analysis of the relative ratio (%) of the amount of blotted protein (ZO-1, ZO-2, and ZO-3) to the amount of GAPDH are shown below the western blotting image. Concerning the degradation of claudin-7, the results of the quantitative analysis of the relative ratio (%) of the amount of the blotted Claudin-7 FG to the amount of GAPDH are shown. These experiments were performed in triplicate. The data are mean +- SD (error bars). *p < 0.01; significance was tested by comparison with the control data (NT).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Increased expression of tight junction proteins in ectocervical cells by L-LA. Protein levels of barrier proteins claudin-1 ( A ), claudin-4 (B ) and tight junction protein-2 (TJP2) ( C ) in ectocervical epithelial cells stimulated apically with 0.3% L-LA pH 3.9 (LA) or low pH media (HCl, pH 3.9) for 1 h followed by lysis of cells at 24 h and assessed by Western blot analysis. Target protein levels were standardised to beta-actin in the same sample. D Expression of claudin-4 was visualised in ectocervical epithelial cells treated as above by immunofluorescence and confocal microscopy. Images from a single experiment representative of n = 4 replicates are shown (left), and mean fluorescence intensity (MFI) of claudin-4 signal intensity was assessed using ImageJ software and expressed relative to untreated samples. All graphs show mean +/- SEM (expressed as fold change compared to untreated cells) from 3 to 5 independent experiments. Significance was assessed by the Mann-Whitney U -test; *, ** p < 0.05 and 0.01, respectively
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
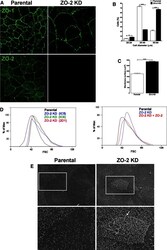
- Experimental details
- FIGURE 1: The absence of ZO-2 altered the cytoarchitecture of epithelial cells. (A) ZO-2 KD cells are bigger than parental MDCK cells. MDCK cells were fixed and processed for immunofluorescence with antibodies against ZO-1 and ZO-2. (B) ZO-2 KD MDCK cells have a bigger diameter than parental cells. The diameter of cells was estimated by comparing in a flow cytometer the FSC signals with those of the reference microspheres. Results from three independent experiments. Statistical analysis done with two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test. * p < 0.05, *** p < 0.001. (C) The amount of membrane surface is bigger in ZO-2 KD than in parental MDCK cells. Membrane surface was estimated by measuring the electrical capacitance in a whole-cell clamp configuration. The membrane surface of 33 parental cells and 36 ZO-2 KD cells was evaluated. Statistical analysis was done with Student's t test, **** p < 0.0001. (D) Left, the FSC of light in a flow cytometer shows that three different clones of ZO-2 KD cells exhibit an increased cell size in comparison to parental cells. Right, the increase in cell size in ZO-2 KD cell clone IC5, evaluated by the FSC of light in a flow cytometer, was partially rescued by expressing a ZO-2 construct with altered shRNA-binding sites. (E) The amount of microvilli varies among cells of the parental MDCK clone (left), but in ZO-2 KD MDCK cells, microvilli density is significantly higher than in parental ce
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
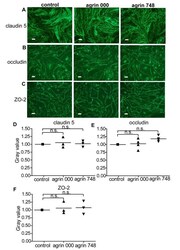
- Experimental details
- Supplementary Figure 5 Agrin 000 and agrin 748 have no influence on the junctional localization of claudin 5, occludin, and ZO-2 in bEnd5 cells. A-C bEnd5 cells cultured on agrin 000, on agrin 748, or under control conditions were stained for claudin-5 ( A ), occludin ( B ) and ZO-2 ( C ) after 48 h in culture. Representative micrographs from 3-4 independent experiments are shown. Bar 50 mum. D-F The gray values of the junctional IF signal of claudin 5 ( D ), occludin ( E ), and ZO-2 ( F ) were measured with ImageJ software. Symbols represent the mean values of each independent experiment ( D , F , n = 3, E n = 4), and the overall means are represented by the horizontal lines . The gray values were normalized to the control condition for every independent experiment (GIF 196 kb)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
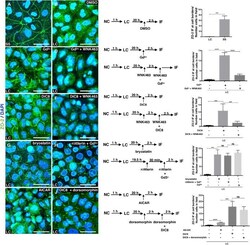
- Experimental details
- FIGURE 1: ZO-2 translocates to the cell borders on activation of the CaSR that through the Galpha q/11 subunit stimulates PKCepsilon that in turn activates WNK proteins and adenylyl cyclase/AMPK. Monolayers were plated at confluent density and incubated in NC media for 24 h (SS) or for 1 h in NC media and then were transferred to LC for 20 h after which they were treated as indicated in the scheme, with 100 muM Gd 3+ , an agonist of CaSR; 4 muM WNK463, an inhibitor of WNK proteins; 0.5 mM DiC8, an activator of conventional and novel PKCs; 200 nM bryostatin, an stimulator of nPKC delta and epsilon; 6 muM rottlerin an inhibitor of nPKCdelta; 4 mM AICAR, a stimulator of AMPK; and 50 muM dorsomorphin, an inhibitor of AMPK. Left panels, immunofluorescence images done with an antibody against ZO-2. Nuclei were stained with DAPI. Bars, 20 mum. Images taken from at least two independent experiments. Middle panels, monolayer treatment scheme. Right panels, quantification of ZO-2 fluorescence staining at the cell borders. Statistical analysis was done with Student's t test *** p < 0.001; **** p < 0.0001; ns, nonsignificant. Results obtained from six optical fields in each experimental condition. Data are from two independent experiments. All the quantitative results in this and the following figures correspond to mean +- SE.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
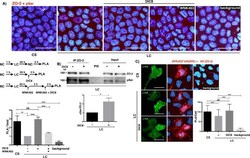
- Experimental details
- FIGURE 2: PKC activation increases the phosphorylation of ZO-2 at serine residues in monolayers cultured in LC media. (A) Monolayers cultured in LC for 20 h were subjected to a CS for 2 h or maintained in LC and treated or not (vehicle only, DMSO 0.25%) for 2 h with 0.5 mM DiC8, or pretreated for 2 h with 4 muM WNK463 and then transferred to media with 4 muM WNK463 plus 0.5 mM DiC8. The PLA was done with a rabbit antibody against ZO-2 and a mouse antibody anti pSer. Background, PLA done in LC monolayers without both primary antibodies. Nuclei stained with DAPI. Bar, 30 mum. Top panel, representative images; middle left panel, schematic design of experiment; bottom left panel, quantitative analysis done using BlobFinder. Statistical analysis done with one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test, ** p < 0.01; **** p < 0.0001. Results were obtained from six optical fields in each experimental condition. Results are from two independent experiments. (B) The amount of phosphorylated serines residues in ZO-2 increased after DiC8 treatment. Western blot of a ZO-2 immunoprecipitate from LC cultured cells, treated or not for 2 h with 0.5 mM DiC8, and blotted against phosphorylated serine residues. Top panel, representative image of three independent experiments; bottom panel, quantitative analysis. Statistical analysis done with Student t test, ** p < 0.01. PIS, preimune serum. (C) MDCK monolayers were transfected with HA-cZO-2, cultured in LC f
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
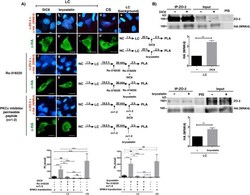
- Experimental details
- FIGURE 3: PKCepsilon activation increases the interaction of ZO-2 with WNK4 in monolayers cultured in LC media. (A) PLA assays done in monolayers cultured in LC or after a CS with a rabbit antibody against ZO-2 and a mouse antibody anti-HA. Monolayers were treated or not for 2 h with 0.5 mM DiC8 or 200 nM bryostatin. In addition, as indicated, some monolayers were pretreated for 30 min and thereafter for 2 h with 25 nM Ro-318220, or 2 muM of the PKCepsilon inhibitor permeable peptide epsilonv1-2. Cells transfected with WNK4-HA were identified with a mouse antibody anti HA, followed by a secondary goat anti-mouse IgG coupled to Alexa Fluor 488. Background corresponds to LC cultured cells not transfected with WNK4-HA. Bars, 25 mum. Top panel, representative images; bottom panel, quantitative analysis done using BlobFinder. Statistical analysis was done with Kruskal-Wallis test followed by Dunn's multiple comparison test, * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns, nonsignificant. Results obtained from 30 transfected cells per condition derived from two independent experiments. (B) Treatment with DiC8 or bryostatin augments the amount of WNK4 that coimmunoprecipitates with ZO-2. ZO-2 was immunoprecipitated from LC cultured cells transfected with a WNK4-HA construct and treated or not for 2 h with 0.5 mM DiC8 or 200 nM bryostatin. After the SDS-PAGE the resulting membranes were blotted against HA and ZO-2. Results are from three independent experiments. Statistic
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
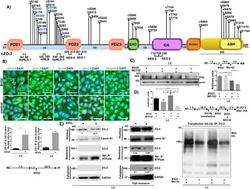
- Experimental details
- FIGURE 4: ZO-2 is sequestered in the cytoplasm by 14-3-3 in the LC condition. (A) cZO-2 and hZO-2 sequences, respectively, have 25 and 22 putative 14-3-3 binding sites, 11 (cZO-2) and 10 (hZO-2) of which are located at the U2 segment. Scheme showing the molecular organization of ZO-2. PDZ, PDZ domain; SH3, Src homologous 3 domain; GK, guanylate kinase domain; Acidic, acidic region; PR, proline-rich segment; ABR, actin binding region; U, unique region; TEL, PDZ binding motif; NLS, nuclear localization signal; NES, nuclear exportation signal; vertical lines, putative 14-3-3 binding sites; blue letters and numbers, putative 14-3-3 binding sites located within an SR motif. (B) The inhibition of 14-3-3 with BV02 induces the appearance of ZO-1 and ZO-2 at the cell borders of cells cultured in LC. MDCK cells were plated at confluent density in NC media and after 1 h were washed 5x with PBS without Ca 2+ and transferred to LC media with 50 muM BV02 or vehicle only (DMSO 0.25%, control). After 20 h the monolayers were fixed and processed for immunofluorescence with antibodies against ZO-1, ZO-2, claudin-1, occludin, and cingulin. Top panel, representative images from three independent experiments. Nuclei were stained in blue with DAPI. Bar, 25 mum. Bottom panel, quantification of ZO-1 and ZO-2 fluorescence staining at the cell borders. Statistical analysis done with Student's t test, **** p < 0.0001. Results obtained from six optical fields in each experimental condition. (C) The cell
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
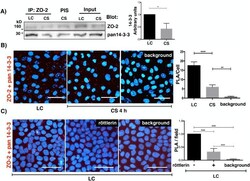
- Experimental details
- FIGURE 5: ZO-2 interacts in the cytoplasm with 14-3-3 in monolayers cultured in LC and this interaction diminishes with the CS. (A) The amount of 14-3-3 that coimmunoprecipitates with ZO-2 diminished after the CS. ZO-2 was immunoprecipitated from monolayers cultured in LC, and after a 4-h CS with a specific antibody, and the membranes were blotted with antibodies against ZO-2 and pan 14-3-3. PIS, preimmune serum. Left panel, representative image of three independent experiments; right panel, quantitative analysis. Statistical analysis was done with Student's t test; * p < 0.05. (B) ZO-2 interaction with 14-3-3 in the cytoplasm of LC cultured cells diminishes with the CS. PLA was done in monolayers cultured in LC and after a 4-h CS with a rabbit antibody against ZO-2 and a mouse antibody anti pan 14-3-3. Nuclei were stained with DAPI. Background, experiment was done without primary antibodies. Bar, 50 mum. Left panel, representative images; right panel, quantitative analysis was done using BlobFinder. Statistical analysis was done with one-way ANOVA followed by Dunnett's multiple comparison test, ** p < 0.01; *** p < 0.0001. Results were obtained from three independent experiments analyzing 60 cells per experimental condition. (C) Monolayers were cultured in LC and treated or not (vehicle only, DMSO 0.25%) for 2 h with 6 muM rottlerin. The PLA was done with a rabbit antibody against ZO-2 and a mouse antibody against pan14-3-3. Background, PLA was done in LC monolayers without
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- FIGURE 6: ZO-2 colocalization with 14-3-3sigma in the cytoplasm of monolayers cultured in LC diminishes with the CS and is accompanied by a transient colocalization at the cell borders. (A) Immunofluorescence observation of ZO-2 and 14-3-3sigma in monolayers cultured in the SS condition, LC condition for 20 h, or 2, 4, and 6 h after a CS. Left panel, representative images; right panel, cytoplasmic PCC. Statistical analysis done with Kruskal-Wallis test followed by Dunn's multiple comparison test; **** p < 0.0001; ns, nonsignificant. Bars, 15 mum. (B) Fluorescence covariance i ndex (FCI = Log10 of PCC at cell periphery/PCC at cytoplasm) was done to compare the frequency of colocalization of 14-3-3sigma with ZO-2 in the cell borders with the colocalization in the cytoplasm. SS, steady state condition. The values of FCI 2, 4, and 6 h after the CS are different from the SS condition since a small but significant proportion of cells exhibited FCI > 0 indicating that in them the colocalization of 14-3-3sigma with ZO-2 was higher at the cell borders than at the cytoplasm. Statistical analysis done with one-way ANOVA followed by Dunnett's multiple comparison test, SS CS (2 h) **** p < 0.0001; SS CS (4 h) **** p < 0.0001; SS CS (6 h).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- FIGURE 7: ZO-2 colocalization with 14-3-3zeta in the cytoplasm of monolayers cultured in LC diminishes with the CS and is accompanied by a transient colocalization at the cell borders. (A) Immunofluorescence observation of ZO-2 and 14-3-3zeta in monolayers cultured in the SS condition, LC condition for 20 h, or 2, 4, and 6 h after a CS. Left panel, representative images; right panel, cytoplasmic Pearson's correlation coefficient. Statistical analysis was done with Kruskal-Wallis test followed by Dunn's multiple comparison test; **** p < 0.0001; ns, nonsignificant. Bars 15 mum. (B) Fluorescence covariance i ndex (FCI = Log10 PCC at cell periphery/PCC at cytoplasm) was done to compare the frequency of colocalization of 14-3-3zeta with ZO-2 in the cell borders with the colocalization in the cytoplasm. SS, steady state condition. The values of FCI 2 and 4 h after the CS are different from the SS condition since a small but significant proportion of cells exhibited FCI > 0 indicating that in them the colocalization of 14-3-3zeta with ZO-2 was higher at the cell borders than at the cytoplasm. Statistical analysis done with Kruskal-Wallis test followed by Dunn's multiple comparison test; SS CS (2 h) **** p < 0.0001; SS CS (4 h) **** p < 0.0001; SS vs. CS (6 h) nonsignificant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
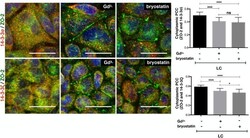
- Experimental details
- FIGURE 8: Activation of the CaSR/PKCepsilon signaling pathway reduces the cytoplasmic colocalization of 14-3-3 and ZO-2. MDCK cells cultured in the LC condition were treated or not for 2 h with 100 muM Gd 3+ or 200 nM bryostatin. Monolayers were processed for immunofluorescence with mouse antibodies against 14-3-3sigma and 14-3-3zeta and rabbit antibodies anti-ZO-2. Left panel, representative images. Bars, 20 mum. Right panel, cytoplasmic PCC was done analyzing 100 cells per condition derived from two independent experiments. Statistical analysis done with Kruskal-Wallis followed by Dunn's multiple comparison test; **** p < 0.0001; * p < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
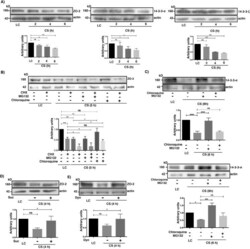
- Experimental details
- FIGURE 9: The cellular content of ZO-2 diminishes after the CS due to lysosome-mediated degradation. (A) The amount of ZO-2, 14-3-3sigma, and 14-3-3zeta diminishes with the CS. Cell lysates were obtained from monolayers cultured in the LC condition for 20 h or 2, 4, and 6 h after a CS. Top panels, representative Western blots; bottom panels, densitometric analysis. Statistical analysis done with One Way ANOVA followed by Dunnett's multiple comparison test. ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001. All the results in this figure correspond to at least three independent experiments. (B) The decay of ZO-2 triggered by the CS can be reversed with cloroquine but not with MG132. Cell lysates were obtained from monolayers cultured in the LC condition for 20 h and incubated or not for 5 additional h with 30 muM cycloheximide (CHX) or transferred to NC media (CS) for 5 h in the presence or absence of 30 muM cycloheximide (CHX) with or without 30 muM MG132 or 50 muM cloroquine. Top panel, representative Western blot; bottom panel, densitometric analysis. Statistical analysis done with one way ANOVA followed by Dunnett's multiple comparison test; ns, not significant; *** p < 0.001; ** p < 0.01; * p < 0.05. (C) The 14-3-3sigma and 14-3-3zeta are degraded in the proteosome and not in the lysosome during the CS. Cell lysates were obtained from monolayers cultured in the LC condition for 20 h or transferred to NC media (CS) for 6 h in the presence or absence of 30 muM MG1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
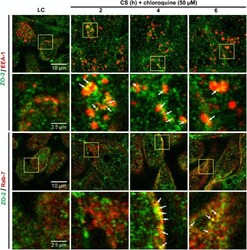
- Experimental details
- FIGURE 10: ZO-2 is endocyted and reaches late endosomes during the CS. Monolayers incubated in the LC condition or transferred to NC media for different periods of time were processed for immunofluorescence with antibodies against ZO-2, the early endosomal marker EEA-1 and the late endosomal marker Rab7. Representative images are from three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
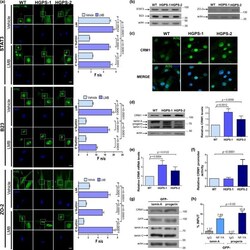
- Experimental details
- Figure 1 Mislocalization of NES-containing proteins is caused by progerin-mediated overexpression of CRM1. (a) Wild-type (WT) and HGPS cells, treated for 24 hr with 10 nM LMB or vehicle alone, were immunolabeled for the indicated proteins (bar, 10 um). The Fn/c ratio was calculated ( n = 50 cells), and significant differences were determined by Mann-Whitney U test (graphs). (b) Protein levels of STAT3, B23, ZO-2, and actin (loading control) were analyzed, and typical gels from two independent experiments are shown. (c) WT and HGPS cells were immunolabeled for CRM1 and analyzed by CLSM. Typical images from 3 independent assays are shown (bar 10 um). (d) Lysates from WT and HGPS cells were subjected to Western blot using antibodies against CRM1, lamin A/C, or actin (loading control), and relative CRM1 levels were obtained (graph). (e) CRM1 messenger RNA expression was examined by qRT-PCR. (d-e) Significant differences were determined by unpaired t test. (f) WT and HGPS cells were transfected with both Luciferase reporter construct containing the human CRM1 promoter and Renilla luciferase vector, which was used to normalize transfection efficiency. Enzymatic activities were estimated after incubation for 48 hr as described in Methods. Data represent mean +- SEM of three independent experiments (unpaired t test). (g) Lysates from HeLa cells stably transfected to express GFP-lamin A or GFP-progerin were subjected to Western blotting using antibodies against CRM1, GFP, or actin (lo
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA