MA4400
antibody from Invitrogen Antibodies
Targeting: CD44
CD44R, CSPG8, HCELL, IN, MC56, MDU2, MDU3, MIC4, Pgp1
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Blocking/Neutralizing
Blocking/Neutralizing Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Immunocytochemistry [5]
- Flow cytometry [2]
- Other assay [7]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA4400 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD44 Monoclonal Antibody (Hermes-1)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA4400 targets CD44 in flow cytometry, immunohistochemistry, immunoprecipitation, ICC/IF and neutralization applications and shows reactivity with bovine, goat, human, ovine, and rabbit samples. The MA4400 immunogen is 80 - 95 kDa lymphocyte surface glycoprotein H-CAM (CD44). This antibody is produced by injecting Rat IgG secreting hybridoma cells into the peritoneum of mice. The resulting ascites is collected from the mice and the antibody is purified. This product has been tested for endotoxins by limulus amoebocyte lysate (LAL) assay and contains an endotoxin concentration of less than or equal to 10 endotoxin units per milligram (EU/mg).
- Reactivity
- Human, Mouse, Bovine, Goat, Rabbit
- Host
- Rat
- Isotype
- IgG
- Antibody clone number
- Hermes-1
- Vial size
- 200 μg
- Concentration
- 1.0 mg/mL
- Storage
- -20°C
Submitted references A single-cell atlas of the normal and malformed human brain vasculature.
Label2label: training a neural network to selectively restore cellular structures in fluorescence microscopy.
Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation.
Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis.
Respiratory Syncytial Virus Infection of Human Lung Fibroblasts Induces a Hyaluronan-Enriched Extracellular Matrix That Binds Mast Cells and Enhances Expression of Mast Cell Proteases.
ADP-ribosylation factor-like 4A interacts with Robo1 to promote cell migration by regulating Cdc42 activation.
Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo.
Epithelial-Mesenchymal Transition Directs Stem Cell Polarity via Regulation of Mitofusin.
Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration.
Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin.
Engineered Healing of Avascular Meniscus Tears by Stem Cell Recruitment.
Cancer stem cell and its niche in malignant progression of oral potentially malignant disorders.
Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D.
The metalloprotease-disintegrin ADAM8 contributes to temozolomide chemoresistance and enhanced invasiveness of human glioblastoma cells.
Increased cerebrospinal fluid osteopontin levels and its involvement in macrophage infiltration in neuromyelitis optica.
VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice.
PKC-induced stiffening of hyaluronan/CD44 linkage; local force measurements on glioma cells.
EGF AND TGF-alpha motogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms.
Fragmented hyaluronan induces transcriptional up-regulation of the multidrug resistance-1 gene in CD4+ T cells.
Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein.
Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices.
Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620).
Heterotypic cell-cell adhesion of human mast cells to fibroblasts.
Heterotypic cell-cell adhesion of human mast cells to fibroblasts.
Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors.
Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types.
A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man.
Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, Chen LQ, Wu D, Catapano JS, Raygor K, Narsinh K, Kim H, Weinsheimer S, Cooke DL, Walcott BP, Lawton MT, Gupta N, Zlokovic BV, Chang EF, Abla AA, Lim DA, Nowakowski TJ
Science (New York, N.Y.) 2022 Mar 4;375(6584):eabi7377
Science (New York, N.Y.) 2022 Mar 4;375(6584):eabi7377
Label2label: training a neural network to selectively restore cellular structures in fluorescence microscopy.
Kölln LS, Salem O, Valli J, Hansen CG, McConnell G
Journal of cell science 2022 Feb 1;135(3)
Journal of cell science 2022 Feb 1;135(3)
Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation.
Kong CS, Ordoñez AA, Turner S, Tremaine T, Muter J, Lucas ES, Salisbury E, Vassena R, Tiscornia G, Fouladi-Nashta AA, Hartshorne G, Brosens JJ, Brighton PJ
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2021 Apr;35(4):e21336
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2021 Apr;35(4):e21336
Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis.
Ko JH, Kim HJ, Jeong HJ, Lee HJ, Oh JY
Cell reports 2020 Mar 17;30(11):3806-3820.e6
Cell reports 2020 Mar 17;30(11):3806-3820.e6
Respiratory Syncytial Virus Infection of Human Lung Fibroblasts Induces a Hyaluronan-Enriched Extracellular Matrix That Binds Mast Cells and Enhances Expression of Mast Cell Proteases.
Reeves SR, Barrow KA, Rich LM, White MP, Shubin NJ, Chan CK, Kang I, Ziegler SF, Piliponsky AM, Wight TN, Debley JS
Frontiers in immunology 2019;10:3159
Frontiers in immunology 2019;10:3159
ADP-ribosylation factor-like 4A interacts with Robo1 to promote cell migration by regulating Cdc42 activation.
Chiang TS, Lin MC, Tsai MC, Chen CH, Jang LT, Lee FS
Molecular biology of the cell 2019 Jan 1;30(1):69-81
Molecular biology of the cell 2019 Jan 1;30(1):69-81
Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo.
Learn GD, McClellan PE, Knapik DM, Cumsky JL, Webster-Wood V, Anderson JM, Gillespie RJ, Akkus O
Journal of biomedical materials research. Part B, Applied biomaterials 2019 Aug;107(6):1864-1876
Journal of biomedical materials research. Part B, Applied biomaterials 2019 Aug;107(6):1864-1876
Epithelial-Mesenchymal Transition Directs Stem Cell Polarity via Regulation of Mitofusin.
Wu MJ, Chen YS, Kim MR, Chang CC, Gampala S, Zhang Y, Wang Y, Chang CY, Yang JY, Chang CJ
Cell metabolism 2019 Apr 2;29(4):993-1002.e6
Cell metabolism 2019 Apr 2;29(4):993-1002.e6
Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration.
D'Agostino G, Saporito A, Cecchinato V, Silvestri Y, Borgeat A, Anselmi L, Uguccioni M
British journal of anaesthesia 2018 Oct;121(4):962-968
British journal of anaesthesia 2018 Oct;121(4):962-968
Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin.
Oyama M, Kariya Y, Kariya Y, Matsumoto K, Kanno M, Yamaguchi Y, Hashimoto Y
The Biochemical journal 2018 May 9;475(9):1583-1595
The Biochemical journal 2018 May 9;475(9):1583-1595
Engineered Healing of Avascular Meniscus Tears by Stem Cell Recruitment.
Tarafder S, Gulko J, Sim KH, Yang J, Cook JL, Lee CH
Scientific reports 2018 May 25;8(1):8150
Scientific reports 2018 May 25;8(1):8150
Cancer stem cell and its niche in malignant progression of oral potentially malignant disorders.
Surendran S, Siddappa G, Mohan A, Hicks W Jr, Jayaprakash V, Mimikos C, Mahri M, Almarzouki F, Morrell K, Ravi R, Govindan S, Sushma CN, Raghavan N, Birur P, Ilayaraja J, Merzianu M, Reid M, Suresh A, Kuriakose MA
Oral oncology 2017 Dec;75:140-147
Oral oncology 2017 Dec;75:140-147
Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D.
Ozdemir T, Fowler EW, Liu S, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S, Jia X
ACS biomaterials science & engineering 2016 Dec 12;2(12):2217-2230
ACS biomaterials science & engineering 2016 Dec 12;2(12):2217-2230
The metalloprotease-disintegrin ADAM8 contributes to temozolomide chemoresistance and enhanced invasiveness of human glioblastoma cells.
Dong F, Eibach M, Bartsch JW, Dolga AM, Schlomann U, Conrad C, Schieber S, Schilling O, Biniossek ML, Culmsee C, Strik H, Koller G, Carl B, Nimsky C
Neuro-oncology 2015 Nov;17(11):1474-85
Neuro-oncology 2015 Nov;17(11):1474-85
Increased cerebrospinal fluid osteopontin levels and its involvement in macrophage infiltration in neuromyelitis optica.
Kariya Y, Kariya Y, Saito T, Nishiyama S, Honda T, Tanaka K, Yoshida M, Fujihara K, Hashimoto Y
BBA clinical 2015 Jun;3:126-34
BBA clinical 2015 Jun;3:126-34
VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice.
Ferjančič Š, Gil-Bernabé AM, Hill SA, Allen PD, Richardson P, Sparey T, Savory E, McGuffog J, Muschel RJ
Blood 2013 Apr 18;121(16):3289-97
Blood 2013 Apr 18;121(16):3289-97
PKC-induced stiffening of hyaluronan/CD44 linkage; local force measurements on glioma cells.
Lamontagne CA, Grandbois M
Experimental cell research 2008 Jan 15;314(2):227-36
Experimental cell research 2008 Jan 15;314(2):227-36
EGF AND TGF-alpha motogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms.
Ellis IR, Schor AM, Schor SL
Experimental cell research 2007 Feb 15;313(4):732-41
Experimental cell research 2007 Feb 15;313(4):732-41
Fragmented hyaluronan induces transcriptional up-regulation of the multidrug resistance-1 gene in CD4+ T cells.
Tsujimura S, Saito K, Kohno K, Tanaka Y
The Journal of biological chemistry 2006 Dec 8;281(49):38089-97
The Journal of biological chemistry 2006 Dec 8;281(49):38089-97
Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein.
Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R
American journal of human genetics 2000 Mar;66(3):859-72
American journal of human genetics 2000 Mar;66(3):859-72
Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices.
Maaser K, Wolf K, Klein CE, Niggemann B, Zänker KS, Bröcker EB, Friedl P
Molecular biology of the cell 1999 Oct;10(10):3067-79
Molecular biology of the cell 1999 Oct;10(10):3067-79
Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620).
Kubens BS, Zänker KS
Cancer letters 1998 Sep 11;131(1):55-64
Cancer letters 1998 Sep 11;131(1):55-64
Heterotypic cell-cell adhesion of human mast cells to fibroblasts.
Trautmann A, Feuerstein B, Ernst N, Bröcker EB, Klein CE
Archives of dermatological research 1997 Mar;289(4):194-203
Archives of dermatological research 1997 Mar;289(4):194-203
Heterotypic cell-cell adhesion of human mast cells to fibroblasts.
Trautmann A, Feuerstein B, Ernst N, Bröcker EB, Klein CE
Archives of dermatological research 1997 Mar;289(4):194-203
Archives of dermatological research 1997 Mar;289(4):194-203
Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors.
Picker LJ, De los Toyos J, Telen MJ, Haynes BF, Butcher EC
Journal of immunology (Baltimore, Md. : 1950) 1989 Mar 15;142(6):2046-51
Journal of immunology (Baltimore, Md. : 1950) 1989 Mar 15;142(6):2046-51
Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types.
Picker LJ, Nakache M, Butcher EC
The Journal of cell biology 1989 Aug;109(2):927-37
The Journal of cell biology 1989 Aug;109(2):927-37
A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man.
Jalkanen ST, Bargatze RF, Herron LR, Butcher EC
European journal of immunology 1986 Oct;16(10):1195-202
European journal of immunology 1986 Oct;16(10):1195-202
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
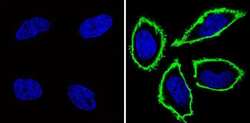
- Experimental details
- Immunofluorescent analysis of CD44 (green) showing staining in the membrane of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CD44 monoclonal antibody (Product # MA4400) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
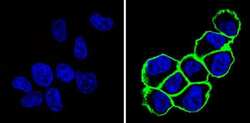
- Experimental details
- Immunofluorescent analysis of CD44 (green) showing staining in the membrane of MDA-MB-231 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CD44 monoclonal antibody (Product # MA4400) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
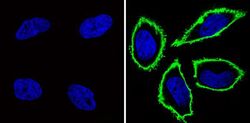
- Experimental details
- Immunofluorescent analysis of CD44 (green) showing staining in the membrane of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CD44 monoclonal antibody (Product # MA4400) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
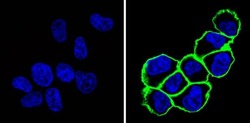
- Experimental details
- Immunofluorescent analysis of CD44 (green) showing staining in the membrane of MDA-MB-231 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CD44 monoclonal antibody (Product # MA4400) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
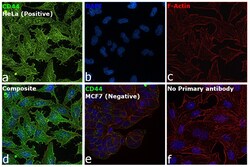
- Experimental details
- Immunofluorescence analysis of CD44 was performed using 70 % confluent log phase HeLa cells. The cells were fixed with 4 % paraformaldehyde for 10 minutes, permeabilized with 0.1 % Triton™ X-100 for 15 minutes, and blocked with 2 % BSA for 45 minutes at room temperature. The cells were labeled with CD44 Monoclonal Antibody (Hermes-1) (Product # MA4400, 1:100) in 0.1 % BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rat IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 488 (Product # A48269, 1:2,000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cell membrane like localization. Panel e represents no expression in MCF7. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
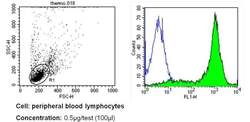
- Experimental details
- Flow cytometry analysis of CD44 in PBMC cells (green) compared to an isotype control (blue). Human blood was collected, combined with a hydrophilic polysaccharide, centrifuged, transferred to a conical tube and washed with PBS. 50 µL of cell solution was added to each tube at a dilution of 2x10^7 cells/mL, followed by the addition of 50 µL of isotype control and primary antibody (Product # MA4400) at a dilution of 0.5 µg/test. Cells were incubated for 30 min at 4ºC and washed with a cell buffer, followed by incubation with a DyLight 488-conjugated secondary antibody for 30 min at 4ºC in the dark. FACS analysis was performed using 400 µL of cell buffer.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
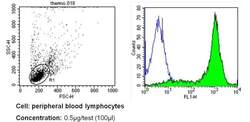
- Experimental details
- Flow cytometry analysis of CD44 in PBMC cells (green) compared to an isotype control (blue). Human blood was collected, combined with a hydrophilic polysaccharide, centrifuged, transferred to a conical tube and washed with PBS. 50 µL of cell solution was added to each tube at a dilution of 2x10^7 cells/mL, followed by the addition of 50 µL of isotype control and primary antibody (Product # MA4400) at a dilution of 0.5 µg/test. Cells were incubated for 30 min at 4ºC and washed with a cell buffer, followed by incubation with a DyLight 488-conjugated secondary antibody for 30 min at 4ºC in the dark. FACS analysis was performed using 400 µL of cell buffer.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
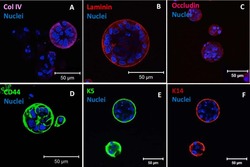
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
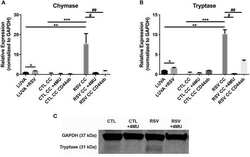
- Experimental details
- Figure 5 Gene expression of mast cell proteases by LUVA cells co-cultured (CC) with HLFs with or without RSV infection for 48 h (RSV CC and CTL CC, respectively). (A) LUVA cells co-cultured with RSV-infected HLFs demonstrated an increased expression of chymase mRNA compared to LUVA cells alone ( ** P = 0.01) and LUVA cells co-cultured with uninfected HLFs ( *** P = 0.002). RSV infection of LUVA cells alone did lead to a small increase in chymase expression at baseline ( * 1.7-fold, P = 0.03). Treatment with either 4-MU or neutralizing antibody to CD44 significantly decreased chymase expression by LUVA cells co-cultured with RSV-infected HLFs ( # P = 0.02; ## P = 0.01, respectively). (B) Expression of tryptase by LUVA cells co-cultured with RSV-infected HLFs was increased compared to LUVA cells alone ( ** P = 0.01) and LUVA cells co-cultured with uninfected HLFs ( *** P = 0.002). RSV infection of LUVA cells alone led to a slight increase in tryptase expression at baseline ( * 1.7-fold, P = 0.03). Treatment with either 4-MU or neutralizing antibody to CD44 decreased the expression of tryptase by LUVA cells co-cultured with RSV-infected HLFs ( # P = 0.02; ## P = 0.01, respectively). (C) Tryptase protein assayed by western blot confirmed a similar pattern as the gene expression analysis. Gene expression was normalized to GAPDH and is shown as normalized mean +- SD relative to control LUVA cells for N = 3-6 replicates/group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
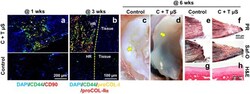
- Experimental details
- Figure 7 Rabbit meniscus healing in vivo by application of fibrin-loaded with CTGF and TGFbeta3 uS. By 1 wk post-op, the recruitment of CD44 + /CD90 + cells into the surgically created meniscus tears was observed with delivery of CTGF and TGFbeta3 uS in contrast to control ( a ) (arrow heads indicate remaining PLGA uS). The recruited CD44 + /CD90 + cells undergo differentiation into proCOL-I + /proCOL-Iialpha + fibrochondrocyte-like cells by 3 wks ( b ) (HR: healing region). CTGF-loaded fibrin with TGFbeta3 uS (C + T uS) successfully enhanced avascular meniscus healing ( c , d ) (Yellow arrows indicate the tear sites), supported by PR ( e ) and Saf-O staining ( f ). There were no noticeable damage on the articular surfaces both with the control ( g ) and C + T uS groups ( h ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
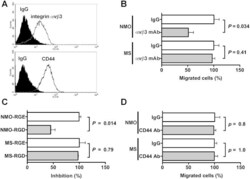
- Experimental details
- Fig. 5 OPN in NMO CSF promotes macrophage motility through integrin alphavbeta3. (A) Expression levels of integrin alphavbeta3 and CD44 on the surface of THP-1 cells were examined using FACS analysis. Prior to analysis, the cells were incubated with either the indicated Abs or control IgG, followed by incubation with Alexa Fluor-conjugated secondary Abs, as described in Materials and Methods. Inhibitory effect of 20 mug/ml integrin alphavbeta3 mAb (clone LM609, B), 100 muM GRGDSP peptide (RGD, C), and 20 mug/ml CD44 mAb (clone Hermes-1, D) on NMO or MS CSF-induced cell motility. The relative number of migrated cells to the lower surface of the membrane in the presence of 20 mug/ml control IgG (IgG, B and D) or 100 muM control GRGESP peptide (RGE, C) was taken as 100%. We used a two-tailed Student's t-test to assess differences. Data are means +- SDs from two or three independent experiments with triplicate data points per experiment. Other experimental conditions are described in the Materials and methods section.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
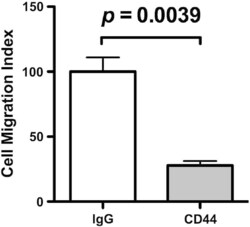
- Experimental details
- Fig. S1 Inhibitory effect of anti-CD44 mAb on MDA-MB231 cell motility. Two hundred microliters of 1 x 10 5 cells/ml human breast cancer cell line MDA-MB231 cells (2 x 10 4 cells in serum-free DMEM) were incubated with 10 mug/ml anti-CD44 mAb (clone Hermes-1) or control IgG. After 20 min incubation at room temperature, the cells were inoculated into each upper well of a chamber, and 750 mul of 10% serum containing DMEM medium was added to the lower well of a chamber. After 18 h incubation, the fixed and stained cells on the lower side of a membrane were photographed using a phase contrast microscope, and the numbers of migrated cells were counted. The relative number of migrated cells to the lower surface of the membrane in the presence of control IgG was taken as 100%. Data are represented as the mean +- SD from three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
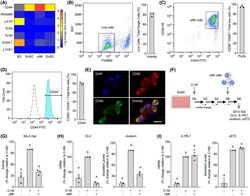
- Experimental details
- FIGURE 2 Decidual-uNK cell co-cultures. A, Heatmap showing relative expression of genes coding putative HA receptors in different endometrial cell types in vivo. EC, endothelial cells; EnEC, endometrial epithelial cells. B-D, Flow cytometric analysis. B, Viability of uNK cells following MACS isolation was determined by FV660 exclusion. C, Purity of uNK cells after MACS isolation was determined by gating cells for CD56 and CD45. D, Histogram depicting CD56 + /CD44 + uNK cells after MACS isolation ( filled histogram ) vs negative control ( open histogram ). Representative dot plots or histogram are shown together with bar graphs (mean +- SEM) showing individual data points of three biological repeat experiments. E, Cytospin preparations of isolated uNK cells stained for DAPI (nucleus), CD56 and CD44. Co-localization of CD56 and CD44 is shown in bottom right panel. Scale = 100 uM. F, Schematic representation of the decidual-uNK cell co-culture killing assay. G-I, Analysis of SA-beta-Gal activity (G), senescent decidual cell markers (H), and decidual markers (I) in decidualizing EnSC co-cultured with or without uNK cells. The data show relative change to the indicated measurements in three biological repeat experiments compared to cultures decidualized with C+M for 8 days in the absence of uNK cells. Different letters above the bar graphs (mean +- SEM) indicate statistical differences ( P < .05) between groups (one-way ANOVA and Dunnett's multiple comparison test)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
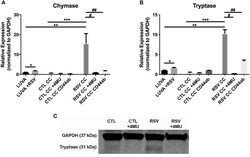
- Experimental details
- Figure 5 Gene expression of mast cell proteases by LUVA cells co-cultured (CC) with HLFs with or without RSV infection for 48 h (RSV CC and CTL CC, respectively). (A) LUVA cells co-cultured with RSV-infected HLFs demonstrated an increased expression of chymase mRNA compared to LUVA cells alone ( ** P = 0.01) and LUVA cells co-cultured with uninfected HLFs ( *** P = 0.002). RSV infection of LUVA cells alone did lead to a small increase in chymase expression at baseline ( * 1.7-fold, P = 0.03). Treatment with either 4-MU or neutralizing antibody to CD44 significantly decreased chymase expression by LUVA cells co-cultured with RSV-infected HLFs ( # P = 0.02; ## P = 0.01, respectively). (B) Expression of tryptase by LUVA cells co-cultured with RSV-infected HLFs was increased compared to LUVA cells alone ( ** P = 0.01) and LUVA cells co-cultured with uninfected HLFs ( *** P = 0.002). RSV infection of LUVA cells alone led to a slight increase in tryptase expression at baseline ( * 1.7-fold, P = 0.03). Treatment with either 4-MU or neutralizing antibody to CD44 decreased the expression of tryptase by LUVA cells co-cultured with RSV-infected HLFs ( # P = 0.02; ## P = 0.01, respectively). (C) Tryptase protein assayed by western blot confirmed a similar pattern as the gene expression analysis. Gene expression was normalized to GAPDH and is shown as normalized mean +- SD relative to control LUVA cells for N = 3-6 replicates/group.
 Explore
Explore Validate
Validate Learn
Learn