Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-41533 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- RIP3 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Sequence homology: Chimpanzee (100%). This antibody is not suitable for testing of mouse RIP3. Immunogen sequence: QEGPKDPEAW SRPQ
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references TRIM21, a New Component of the TRAIL-Induced Endogenous Necrosome Complex.
Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury.
Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis.
Simoes Eugénio M, Faurez F, Kara-Ali GH, Lagarrigue M, Uhart P, Bonnet MC, Gallais I, Com E, Pineau C, Samson M, Le Seyec J, Dimanche-Boitrel MT
Frontiers in molecular biosciences 2021;8:645134
Frontiers in molecular biosciences 2021;8:645134
Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury.
Martens S, Jeong M, Tonnus W, Feldmann F, Hofmans S, Goossens V, Takahashi N, Bräsen JH, Lee EW, Van der Veken P, Joossens J, Augustyns K, Fulda S, Linkermann A, Song J, Vandenabeele P
Cell death & disease 2017 Jun 29;8(6):e2904
Cell death & disease 2017 Jun 29;8(6):e2904
Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis.
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW, Park EJ, Chun KH, Song J
Nature communications 2012;3:978
Nature communications 2012;3:978
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
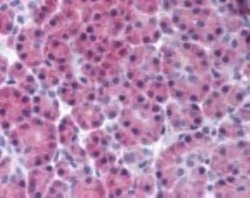
- Experimental details
- Immunohistochemical analysis of RIP3 in Human pancreas. Samples were incubated in RIP3 polyclonal antibody (Product # PA1-41533) using a dilution of 10 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
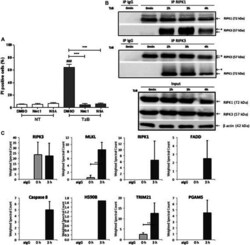
- Experimental details
- FIGURE 1 TRAIL/z-VAD-fmk/Birinapant (TzB) induces necroptosis and necrosome formation in HT29 cells. (A) HT29 cells were pretreated with vehicle (DMSO 0.1%), Necrostatin-1 (Nec1, 10 uM) or Necrosulfonamide (NSA, 1 uM) for 1 h. Cells were then treated with TzB (in grey) or not (NT, in white) for 24 h. Percentage of PI positive dead cells were measured by flow cytometry (mean +- SD, n = 3). (###), p < 0.001 compared treated cells to NT cells; (***), p < 0.001 compared treated conditions. (B) HT29 cells were treated or not (0 min) with TRAIL-SK (500 ng/ml), z-VAD-fmk (25 uM) and Birinapant (Bp, 1 uM) for the indicated times. Four mg of cell lysates were immunoprecipitated (IP) with RIPK1 or RIPK3 antibody or control IgG. The RIPK1 or RIPK3 immunocomplexes were analyzed by western blot. *: an upper band for RIPK3 or a smear for RIPK1. Anti-human beta-actin antibody was used as protein loading control. Representative data of three independent experiments. (C) Label-free quantification of protein of interest by mass spectrometry. Weighted spectral counts were calculated (means +- SD, n = 2) from the analysis of two different pools of eight RIPK3 immunoprecipitates for RIPK3, MLKL, RIPK1, FADD, Caspase 8, HS90B, TRIM21, and PGAM5 observed in IgG control (aIgG) and at times 0 and 3 h post-stimulation. (**) p < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
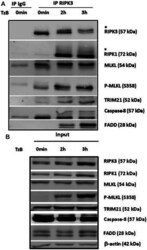
- Experimental details
- FIGURE 2 RIPK1, MLKL, P-MLKL, and TRIM21 are recruited to the endogenous RIPK3-dependent necrosome. HT29 cells were treated or not (0 min) with TRAIL-SK (500 ng/ml), z-VAD-fmk (25 uM), and Birinapant (Bp, 1 uM) (TzB) for the indicated times. Four mg of cell lysates were immunoprecipitated (IP) with RIPK3 antibody or control IgG as described in materials and methods. Upper panel: RIPK3 immunocomplexes were analyzed for indicated proteins by immunoblotting. *: an upper band for RIPK3 or a smear for RIPK1. Lower panel: expression of the indicated proteins was analyzed in the total cell lysates (input) by immunoblotting. Anti-human beta-actin antibody was used as protein loading control. Representative data of three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
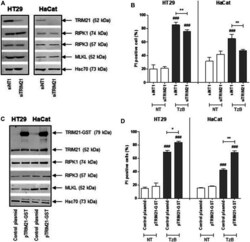
- Experimental details
- FIGURE 3 Downregulation or overexpression of TRIM21 expression negatively or positively regulates TRAIL/z-VAD-fmk/Birinapant (TzB)-induced necroptosis, respectively. (A) HT29 and HaCat cells were transiently transfected with siTRIM21 or siNT1 (negative control). Immunoblot analysis of TRIM21, RIPK1, RIPK3, and MLKL expressions was carried out 72 h post-transfection. Anti-human Hsc70 antibody was used as protein loading control. Representative data of three independent experiments. (B) Forty eight hours after siRNA transfections, HT29, and HaCat cells were treated (in grey) or not (in white) with TRAIL-SK (100 ng/ml), z-VAD-fmk (25 uM) and Birinapant (Bp, 1 uM) (TzB) for 24 h. Percentages of necrotic cells (propidium iodide (PI) positive) were estimated (means +- SD, n = 3). (C) HT29 and HaCat cells were transiently transfected with pTRIM21-GST or control plasmid (used as a negative control). Immunoblot analysis of TRIM21, RIPK1, RIPK3, and MLKL expressions was carried out 72 h after transfection. Anti-human Hsc70 antibody was used as protein loading control. Representative data of three independent experiments. (D) Forty eight hours after transfection, HT29, and HaCat cells were treated (in grey) or not (in white) with TRAIL-SK (100 ng/ml), z-VAD-fmk (25 uM), and Birinapant (Bp, 1 uM) (TzB) for 24 h. Percentages of necrotic cells (propidium iodide (PI) positive) were estimated (means +- SD, n = 3). (###), p < 0.001 compared treated cells to NT cells; (*), p < 0.05, and (**),
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
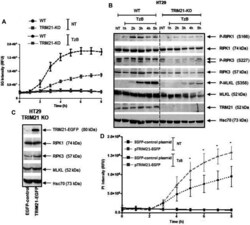
- Experimental details
- FIGURE 4 TRIM21-KO HT29 cells are more resistant to TRAIL/z-VAD-fmk/Birinapant (TzB)-induced necroptosis and their sensitivity can be rescued by TRIM21 re-expression. (A) WT (black lines) or TRIM21-KO (grey lines) HT29 cells were treated (dashed lines) or not (solid lines) with TRAIL-SK (100 ng/ml), z-VAD-fmk (25 uM), and Birinapant (Bp, 1 uM) (TzB) during 8 h. Necroptosis was evaluated by measuring SYTOX(tm) Green (SG) intensity (RFU) as described in materials and methods. Representative data of three independent experiments. (B) WT and TRIM21-KO HT29 cells were treated with TzB during the indicated times. Immunoblot analysis of TRIM21, RIPK1, RIPK3, MLKL, and phosphorylated forms of RIPK1, RIPK3, and MLKL were evaluated. Anti-human Hsc70 antibody was used as protein loading control. Representative data of three independent experiments. (C) TRIM21-KO HT29 cells were transfected with EGFP-control plasmid or TRIM21-EGFP plasmid for 48 h and approximately 40% of cells were GFP positive for each transfection conditions. Immunoblot analysis of TRIM21, RIPK1, RIPK3 and, MLKL expressions was carried out 48 h after transfection. Anti-human Hsc70 antibody was used as protein loading control. (D) After transfection with the EGFP-control plasmid (black lines) or with the pTRIM21-EGFP (grey lines), cells were treated or not (NT, solid lines) with TRAIL-SK (100 ng/ml), z-VAD-fmk (25 uM), and Birinapant (Bp, 1 uM) (TzB, dashed lines) during 8 h. Necroptosis was evaluated by measuring prop
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry