Antibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Flow cytometry [1]
- Chromatin Immunoprecipitation [2]
- Other assay [20]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 44-244G - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-SMAD2 (Ser465, Ser467) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Storage
- -20°C
Submitted references Fucoidan-Mediated Inhibition of Fibrotic Properties in Oral Submucous Fibrosis via the MEG3/miR-181a/Egr1 Axis.
Periosteal CD68(+) F4/80(+) Macrophages Are Mechanosensitive for Cortical Bone Formation by Secretion and Activation of TGF-β1.
Targeting TGF-β for treatment of osteogenesis imperfecta.
TGF-β1 increases permeability of ciliated airway epithelia via redistribution of claudin 3 from tight junction into cell nuclei.
Differential Role of Smad2 and Smad3 in the Acquisition of an Endovascular Trophoblast-Like Phenotype and Preeclampsia.
Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation.
Role of sialidase Neu3 and ganglioside GM3 in cardiac fibroblasts activation.
Cholesterol Pathway Inhibition Induces TGF-β Signaling to Promote Basal Differentiation in Pancreatic Cancer.
The Immunosuppressive Microenvironment in BRCA1-IRIS-Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages.
Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling.
The neovascularization effect of dedifferentiated fat cells.
A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling.
Aggressive Mammary Cancers Lacking Lymphocytic Infiltration Arise in Irradiated Mice and Can Be Prevented by Dietary Intervention.
Prostate epithelial-specific expression of activated PI3K drives stromal collagen production and accumulation.
Pancreatic Ductal Deletion of Hnf1b Disrupts Exocrine Homeostasis, Leads to Pancreatitis, and Facilitates Tumorigenesis.
The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes.
Therapeutic discovery for marrow failure with MDS predisposition using pluripotent stem cells.
Loss of EMILIN-1 Enhances Arteriolar Myogenic Tone Through TGF-β (Transforming Growth Factor-β)-Dependent Transactivation of EGFR (Epidermal Growth Factor Receptor) and Is Relevant for Hypertension in Mice and Humans.
Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells.
A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells.
Myostatin propeptide mutation of the hypermuscular Compact mice decreases the formation of myostatin and improves insulin sensitivity.
Oncogenic Kras drives invasion and maintains metastases in colorectal cancer.
Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi.
Conditional ablation of TGF-β signaling inhibits tumor progression and invasion in an induced mouse bladder cancer model.
Effects of a novel Nodal-targeting monoclonal antibody in melanoma.
BMP signaling controls muscle mass.
The significance of a Cripto-1 positive subpopulation of human melanoma cells exhibiting stem cell-like characteristics.
Fang CY, Chen SH, Huang CC, Liao YW, Chao SC, Yu CC
Pharmaceuticals (Basel, Switzerland) 2022 Jul 5;15(7)
Pharmaceuticals (Basel, Switzerland) 2022 Jul 5;15(7)
Periosteal CD68(+) F4/80(+) Macrophages Are Mechanosensitive for Cortical Bone Formation by Secretion and Activation of TGF-β1.
Deng R, Li C, Wang X, Chang L, Ni S, Zhang W, Xue P, Pan D, Wan M, Deng L, Cao X
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2022 Jan;9(3):e2103343
Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2022 Jan;9(3):e2103343
Targeting TGF-β for treatment of osteogenesis imperfecta.
Song IW, Nagamani SC, Nguyen D, Grafe I, Sutton VR, Gannon FH, Munivez E, Jiang MM, Tran A, Wallace M, Esposito P, Musaad S, Strudthoff E, McGuire S, Thornton M, Shenava V, Rosenfeld S, Huang S, Shypailo R, Orwoll E, Lee B
The Journal of clinical investigation 2022 Apr 1;132(7)
The Journal of clinical investigation 2022 Apr 1;132(7)
TGF-β1 increases permeability of ciliated airway epithelia via redistribution of claudin 3 from tight junction into cell nuclei.
Schilpp C, Lochbaum R, Braubach P, Jonigk D, Frick M, Dietl P, Wittekindt OH
Pflugers Archiv : European journal of physiology 2021 Feb;473(2):287-311
Pflugers Archiv : European journal of physiology 2021 Feb;473(2):287-311
Differential Role of Smad2 and Smad3 in the Acquisition of an Endovascular Trophoblast-Like Phenotype and Preeclampsia.
Brkić J, Dunk C, Shan Y, O'Brien JA, Lye P, Qayyum S, Yang P, Matthews SG, Lye SJ, Peng C
Frontiers in endocrinology 2020;11:436
Frontiers in endocrinology 2020;11:436
Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation.
Yoon JJ, Lee HK, Kim HY, Han BH, Lee HS, Lee YJ, Kang DG
International journal of molecular sciences 2020 Sep 23;21(19)
International journal of molecular sciences 2020 Sep 23;21(19)
Role of sialidase Neu3 and ganglioside GM3 in cardiac fibroblasts activation.
Ghiroldi A, Piccoli M, Creo P, Cirillo F, Rota P, D'Imperio S, Ciconte G, Monasky MM, Micaglio E, Garatti A, Aureli M, Carsana EV, Menicanti L, Pappone C, Anastasia L
The Biochemical journal 2020 Sep 18;477(17):3401-3415
The Biochemical journal 2020 Sep 18;477(17):3401-3415
Cholesterol Pathway Inhibition Induces TGF-β Signaling to Promote Basal Differentiation in Pancreatic Cancer.
Gabitova-Cornell L, Surumbayeva A, Peri S, Franco-Barraza J, Restifo D, Weitz N, Ogier C, Goldman AR, Hartman TR, Francescone R, Tan Y, Nicolas E, Shah N, Handorf EA, Cai KQ, O'Reilly AM, Sloma I, Chiaverelli R, Moffitt RA, Khazak V, Fang CY, Golemis EA, Cukierman E, Astsaturov I
Cancer cell 2020 Oct 12;38(4):567-583.e11
Cancer cell 2020 Oct 12;38(4):567-583.e11
The Immunosuppressive Microenvironment in BRCA1-IRIS-Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages.
Sami E, Paul BT, Koziol JA, ElShamy WM
Cancer research 2020 Mar 1;80(5):1102-1117
Cancer research 2020 Mar 1;80(5):1102-1117
Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling.
Chamberlin T, Thompson V, Hillers-Ziemer LE, Walton BN, Arendt LM
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2020 Jun;34(6):8611-8624
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2020 Jun;34(6):8611-8624
The neovascularization effect of dedifferentiated fat cells.
Watanabe H, Goto S, Kato R, Komiyama S, Nagaoka Y, Kazama T, Yamamoto C, Li Y, Konuma N, Hagikura K, Matsumoto T
Scientific reports 2020 Jun 8;10(1):9211
Scientific reports 2020 Jun 8;10(1):9211
A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling.
Wójcik-Pszczoła K, Chłoń-Rzepa G, Jankowska A, Ślusarczyk M, Ferdek PE, Kusiak AA, Świerczek A, Pociecha K, Koczurkiewicz-Adamczyk P, Wyska E, Pękala E, Gosens R
International journal of molecular sciences 2020 Jun 3;21(11)
International journal of molecular sciences 2020 Jun 3;21(11)
Aggressive Mammary Cancers Lacking Lymphocytic Infiltration Arise in Irradiated Mice and Can Be Prevented by Dietary Intervention.
Omene C, Ma L, Moore J, Ouyang H, Illa-Bochaca I, Chou W, Patel MS, Sebastiano C, Demaria S, Mao JH, Karagoz K, Gatza ML, Barcellos-Hoff MH
Cancer immunology research 2020 Feb;8(2):217-229
Cancer immunology research 2020 Feb;8(2):217-229
Prostate epithelial-specific expression of activated PI3K drives stromal collagen production and accumulation.
Wegner KA, Mueller BR, Unterberger CJ, Avila EJ, Ruetten H, Turco AE, Oakes SR, Girardi NM, Halberg RB, Swanson SM, Marker PC, Vezina CM
The Journal of pathology 2020 Feb;250(2):231-242
The Journal of pathology 2020 Feb;250(2):231-242
Pancreatic Ductal Deletion of Hnf1b Disrupts Exocrine Homeostasis, Leads to Pancreatitis, and Facilitates Tumorigenesis.
Quilichini E, Fabre M, Dirami T, Stedman A, De Vas M, Ozguc O, Pasek RC, Cereghini S, Morillon L, Guerra C, Couvelard A, Gannon M, Haumaitre C
Cellular and molecular gastroenterology and hepatology 2019;8(3):487-511
Cellular and molecular gastroenterology and hepatology 2019;8(3):487-511
The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes.
Arif E, Solanki AK, Srivastava P, Rahman B, Tash BR, Holzman LB, Janech MG, Martin R, Knölker HJ, Fitzgibbon WR, Deng P, Budisavljevic MN, Syn WK, Wang C, Lipschutz JH, Kwon SH, Nihalani D
Kidney international 2019 Jul;96(1):139-158
Kidney international 2019 Jul;96(1):139-158
Therapeutic discovery for marrow failure with MDS predisposition using pluripotent stem cells.
Ruiz-Gutierrez M, Bölükbaşı ÖV, Alexe G, Kotini AG, Ballotti K, Joyce CE, Russell DW, Stegmaier K, Myers K, Novina CD, Papapetrou EP, Shimamura A
JCI insight 2019 Apr 30;5(12)
JCI insight 2019 Apr 30;5(12)
Loss of EMILIN-1 Enhances Arteriolar Myogenic Tone Through TGF-β (Transforming Growth Factor-β)-Dependent Transactivation of EGFR (Epidermal Growth Factor Receptor) and Is Relevant for Hypertension in Mice and Humans.
Carnevale D, Facchinello N, Iodice D, Bizzotto D, Perrotta M, De Stefani D, Pallante F, Carnevale L, Ricciardi F, Cifelli G, Da Ros F, Casaburo M, Fardella S, Bonaldo P, Innocenzi G, Rizzuto R, Braghetta P, Lembo G, Bressan GM
Arteriosclerosis, thrombosis, and vascular biology 2018 Oct;38(10):2484-2497
Arteriosclerosis, thrombosis, and vascular biology 2018 Oct;38(10):2484-2497
Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells.
Caja L, Tzavlaki K, Dadras MS, Tan EJ, Hatem G, Maturi NP, Morén A, Wik L, Watanabe Y, Savary K, Kamali-Moghaddan M, Uhrbom L, Heldin CH, Moustakas A
Oncogene 2018 May;37(19):2515-2531
Oncogene 2018 May;37(19):2515-2531
A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells.
David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C
Oncoimmunology 2017;6(10):e1349589
Oncoimmunology 2017;6(10):e1349589
Myostatin propeptide mutation of the hypermuscular Compact mice decreases the formation of myostatin and improves insulin sensitivity.
Kocsis T, Trencsenyi G, Szabo K, Baan JA, Muller G, Mendler L, Garai I, Reinauer H, Deak F, Dux L, Keller-Pinter A
American journal of physiology. Endocrinology and metabolism 2017 Mar 1;312(3):E150-E160
American journal of physiology. Endocrinology and metabolism 2017 Mar 1;312(3):E150-E160
Oncogenic Kras drives invasion and maintains metastases in colorectal cancer.
Boutin AT, Liao WT, Wang M, Hwang SS, Karpinets TV, Cheung H, Chu GC, Jiang S, Hu J, Chang K, Vilar E, Song X, Zhang J, Kopetz S, Futreal A, Wang YA, Kwong LN, DePinho RA
Genes & development 2017 Feb 15;31(4):370-382
Genes & development 2017 Feb 15;31(4):370-382
Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi.
Margaryan NV, Gilgur A, Seftor EA, Purnell C, Arva NC, Gosain AK, Hendrix MJ, Strizzi L
International journal of molecular sciences 2016 Mar 22;17(3):418
International journal of molecular sciences 2016 Mar 22;17(3):418
Conditional ablation of TGF-β signaling inhibits tumor progression and invasion in an induced mouse bladder cancer model.
Liang Y, Zhu F, Zhang H, Chen D, Zhang X, Gao Q, Li Y
Scientific reports 2016 Jul 5;6:29479
Scientific reports 2016 Jul 5;6:29479
Effects of a novel Nodal-targeting monoclonal antibody in melanoma.
Strizzi L, Sandomenico A, Margaryan NV, Focà A, Sanguigno L, Bodenstine TM, Chandler GS, Reed DW, Gilgur A, Seftor EA, Seftor RE, Khalkhali-Ellis Z, Leonardi A, Ruvo M, Hendrix MJ
Oncotarget 2015 Oct 27;6(33):34071-86
Oncotarget 2015 Oct 27;6(33):34071-86
BMP signaling controls muscle mass.
Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, Stantzou A, Mouisel E, Toniolo L, Ferry A, Stricker S, Goldberg AL, Dupont S, Piccolo S, Amthor H, Sandri M
Nature genetics 2013 Nov;45(11):1309-18
Nature genetics 2013 Nov;45(11):1309-18
The significance of a Cripto-1 positive subpopulation of human melanoma cells exhibiting stem cell-like characteristics.
Strizzi L, Margaryan NV, Gilgur A, Hardy KM, Normanno N, Salomon DS, Hendrix MJ
Cell cycle (Georgetown, Tex.) 2013 May 1;12(9):1450-6
Cell cycle (Georgetown, Tex.) 2013 May 1;12(9):1450-6
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
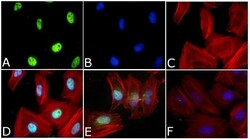
- Experimental details
- Immunofluorescent analysis of Phospho-SMAD2 pSer465/pSer467 Antibody was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were treated with TGF-beta1 (20 ng/mL) for 15 minutes and labeled with Phospho-SMAD2 pSer465/pSer467 Antibody (Product # 44-244G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing Nuclear localization. Panel e showing merged image of untreated cells with less nuclear signals Panel f is a no primary antibody control. The images were captured at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
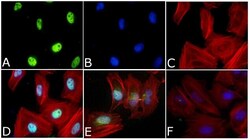
- Experimental details
- Immunofluorescent analysis of Phospho-SMAD2 pSer465/pSer467 Antibody was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were treated with TGF-beta1 (20 ng/mL) for 15 minutes and labeled with Phospho-SMAD2 pSer465/pSer467 Antibody (Product # 44-244G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing Nuclear localization. Panel e showing merged image of untreated cells with less nuclear signals Panel f is a no primary antibody control. The images were captured at 40X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
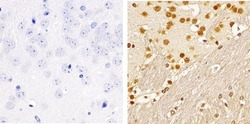
- Experimental details
- Immunohistochemistry analysis of SMAD2 (pSpS465/467) showing staining in the nucleus of paraffin-embedded mouse brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SMAD2 (pSpS465/467) polyclonal antibody (Product # 44-244G) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
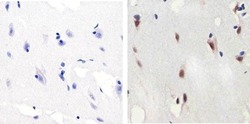
- Experimental details
- Immunohistochemistry analysis of SMAD2 (pSpS465/467)showing staining in the nucleus of paraffin-embedded human brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SMAD2 (pSpS465/467) polyclonal antibody (Product # 44-244G) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
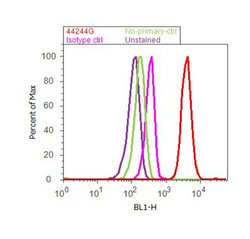
- Flow cytometry analysis of SMAD2 [pSpS465/467] was done on A549 cells treated with TGF beta (20ng/mL, 15 minutes). Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with SMAD2 [pSpS465/467] Rabbit Polyclonal Antibody (44244G, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
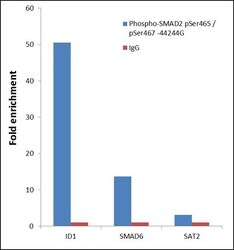
- ChIP- qPCR analysis of SMAD2 pSer465/pSer467 was performed with 10 µL of the Phospho-SMAD2 pSer465/pSer467 Rabbit polyclonal antibody (Product # 44-244G) on sheared chromatin from 2 million Jurkat cells treated with 50 ng/mL of TGF-beta for one hour using the MAGnify™ Chromatin Immunoprecipitation System (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA from each ChIP sample was analyzed by StepOnePlus™ Real-Time PCR System (Product # 4376600) with primers for the promoter of active ID1, SMAD6 gene, used as positive control target, and the SAT2 used as negative control target. Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
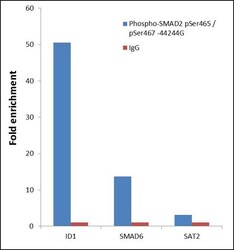
- ChIP- qPCR analysis of SMAD2 pSer465/pSer467 was performed with 10 µL of the Phospho-SMAD2 pSer465/pSer467 Rabbit polyclonal antibody (Product # 44-244G) on sheared chromatin from 2 million Jurkat cells treated with 50 ng/mL of TGF-beta for one hour using the MAGnify™ Chromatin Immunoprecipitation System (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA from each ChIP sample was analyzed by StepOnePlus™ Real-Time PCR System (Product # 4376600) with primers for the promoter of active ID1, SMAD6 gene, used as positive control target, and the SAT2 used as negative control target. Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
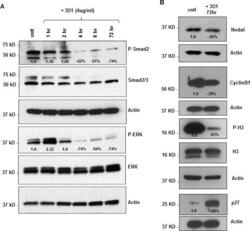
- Experimental details
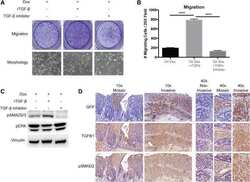
- Figure 4 Effects of anti-Nodal 3D1 antibody on cell signaling and cell cycle related molecules A. Levels of P-Smad2 and P-ERK1/2 are reduced within 4hr of 3D1 mAb treatment (4 mug/ml) in C8161 human melanoma cells compared to IgG treated control. B. After 72 hr of 3D1 mAb treatment there is a reduction of Nodal, Cyclin B1 and P-H3 with a concomitant increase in p27 in C8161 cells compared to IgG treated control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
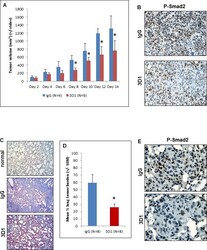
- Experimental details
- Figure 5 In vivo effects of anti-Nodal 3D1 mAb on C8161 human melanoma cells A. Significantly reduced tumor volumes are observed in C8161 Nude mice orthotopic xenografts treated with direct tumor injections of 3D1 mAb versus control IgG. Histograms represent mean values +/- SD. Representative IHC staining in B. shows reduced nuclear expression (activation) of Smad2 in C8161 orthotopic xenograft Nude mouse treated with 3D1 mAb compared to IgG control (20X original magnification). The potential for lung colonization (shown microscopically with H&E staining) of C8161 cells (after systemic introduction) in Nude mice C. (40X original magnification) is significantly reduced in animals treated with IP administration of 3D1 mAb vs IgG control D. Histograms represent mean values +/- SEM. Representative IHC staining in E. shows reduced nuclear expression (activation) of P-Smad2 in C8161 lung colony of a 3D1 mAb treated Nude mouse versus IgG control (63X original magnification). (* P < 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
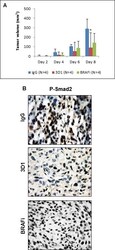
- Experimental details
- Figure 6 In vivo effects of anti-Nodal 3D1 mAb on A375SM human melanoma cells A. After 8 days, mean tumor volume of A375SM orthotopic Nude mice xenografts was significantly smaller in 3D1 mAb treated than in IgG control treated animals. Tumor volumes in dabrafenib (BRAFi) treated animals also showed a trend towards reduced tumor volumes compared to control. Histograms represent mean values +/- SD. B. Representative IHC of P-Smad2 showing nuclear staining in A375SM orthotopic xenografts in Nude mice treated with control IgG, 3D1 mAb or BRAFi (* P < 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
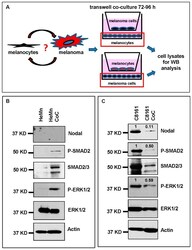
- Experimental details
- Figure 3 Co-culture (CoC) experiments. ( A ) Schematic diagram summarizing the co-culture experiments using HeMn melanocytes and C8161 melanoma cells. Lysates of cells growing on the bottom well (red square) were used for Western blotting; ( B ) Western blot results show increased expression of P-SMAD2 and P-ERK1/2 in HeMn melanocytes co-cultured with human C8161 melanoma cells compared to control HeMn cells culured alone. However, Nodal expression was not detected in HeMn cells grown alone or in co-culture with C8161 melanoma cells; and ( C ) Results from WB analysis show reduced levels of Nodal, P-SMAD2 and P-ERK1/2 in C8161 melanoma cells when co-cultured with HeMn melanocytes. Numbers above WB bands represent densitometric units, normalized to actin loading control, total SMAD2/3 or total ERK1/2, as appropriate, relative to control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
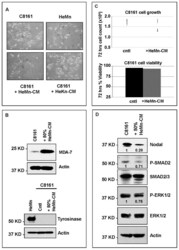
- Experimental details
- Figure 4 Effect of treatment of C8161 melanoma cells with HeMn melanocyte conditioned medium (HeMn-CM). ( A ) C8161 cells treated with HeMn-CM show morphologic changes such as dendritic shape with long cytoplasmic extensions reminiscent of melanocyte differentiation. (40x original magnification); ( B ) Although, Melanoma Differentiation Associated protein 7 (MDA-7) was detected in C8161 cells treated with HeMn-CM, the melanin synthesis associated enzyme tyrosinase was not; ( C ) C8161 cells treated with HeMn-CM showed similar proliferation rate and cell viability as non-treated control C8161 cells making potential toxic effect of HeMn-CM on C8161 unlikely; ( D ) Western blot analysis show that HeMn-CM treated C8161 express lower levels of Nodal, P-SMAD2 and P-ERK1/2 compared to untreated control C8161 cells. Numbers below WB bands represent densitometric units, normalized to actin loading control, total SMAD2/3 or total ERK1/2, as appropriate, relative to control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
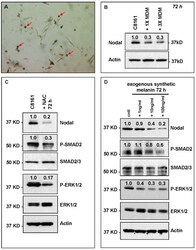
- Experimental details
- Figure 5 HeMn melanocyte derived melanosomes (MDM) affect Nodal expression and signaling components in C8161. ( A ) Microphotograph (40x original magnification) shows MDM containing globules (red arrows) produced by HeMn melanocytes; ( B ) C8161 melanoma cells treated with different concentrations of MDM collected from HeMn melanocytes showed reduction in Nodal expression compared to untreated control C8161 cells. To mimic the anti-oxidant effect of free radical scavengers contained in MDM, C8161 cells were treated with N -acetyl- l -cysteine (NAC) known to generate the anti-oxidant, glutathione; ( C ) Western Blot analysis show that treatment of C8161 melanoma cells with 5 mM NAC resulted in reduced levels of Nodal, P-SMAD and P-ERK1/2 compared to untreated control C8161 cells; ( D ) Treatment of C8161 cells with synthetic melanin, also known to have anti-oxidant properties, resulted in a dose-dependent reduction of Nodal, P-SMAD and P-ERK1/2 levels compared to untreated control C8161 cells. Numbers above WB bands represent densitometric units, normalized to actin loading control, total SMAD2/3 or total ERK1/2, as appropriate, relative to control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
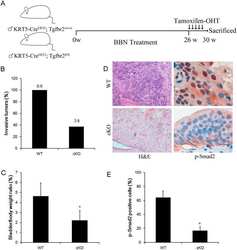
- Pbsn-cre4Prb/+;PI3K GOF/+ mouse prostatic stroma has increased levels of p-SMAD2, a biomarker of TGF-beta activity. (A) Dorsal prostate sections from 4-, 7- and 12-month-old Pbsn-cre4Prb+/+;PI3K GOF/+ (control) and Pbsn-cre4Prb/+;PI3K GOF/+ male mice were stained with an antibody recognizing the phosphorylated form of SMAD2 (p-SMAD2) and DAPI to mark nuclei. Slides were imaged using fluorescence microscopy. (B) The frequency of p-SMAD2-positive stromal cells began to differ between groups starting at 7 months and continuing until at least 12 months of age. (C) Pbsn-cre4Prb/+;PI3K GOF/+ male mice were stained using RNAscope(r) to visualize Tgfb1 mRNA. Representative image of greater Tgfb1 mRNA abundance in the epithelium of Pbsn-cre4Prb/+;PI3K GOF/+ mice. Mean +- SEM of two to four mice. Significant differences among groups: n.s., not significant; * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
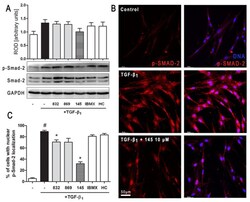
- Experimental details
- Figure 6 Compound 145 limits TGF-beta 1 -induced Smad-2 phosphorylation and nuclear translocation in MRC-5 cells. Lung fibroblasts were pre-incubated with the studied compounds (10 µM) for 1 h, and then TGF-beta 1 (5 ng/mL) was added for 1 h. ( A ) Representative immunoblot and densitometry analysis of p-Smad-2 in MRC-5 ( n = 4). The relative optical density (ROD) signal was normalized to the GAPDH control level. Each bar represents the mean value (+- S.E.M.). ( B ) Immunofluorescence staining for p-Smad-2 protein: MRC-5 were fixed, permeabilized, blocked, and incubated with anti-p-Smad-2 antibody followed by Alexa Fluor 546 conjugated antibody, nuclei were stained with the Hoechst 33342 dye. Microphotographs were taken using Leica DMi8 microscope with Thunder Imaging System, 40x objective, bar = 50 um. ( C ) Cells exhibiting strong nuclear p-Smad-2 signals were counted in 20 randomly selected fields of view and expressed as a percentage fraction in the entire MRC-5 population. The results were considered statistically significant at the p level of 0.05, against the control (#) and TGF-beta 1 (*) conditions.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
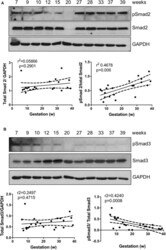
- Experimental details
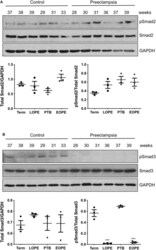
- Figure 1 Smad2 and Smad3 expression in placentas across gestation. Healthy placentas from 6 to 39 weeks of pregnancy were assessed for total and phosphor-(p) Smad2 (A) and Smad3 (B) levels using Western Blotting. Representative blots and linear regression graphs depicting the relationship between their levels and gestational stage. The number on the top of each lane indicates gestational age of the sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
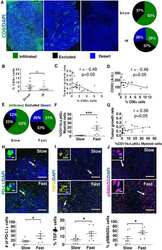
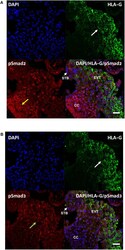
- Figure 2 Differential cellular localization of the active forms of Smad2 (A) and Smad3 (B) in anchoring villi. Immunofluorescence staining was performed on 10-14 week placentas ( n = 3) using antibodies against pSmad2 or pSmad3, as well as HLA-G to identify EVTs. Nuclei were stained with DAPI. Representative pictures from a 12-week placenta are shown. Nuclear staining for pSmad2 was more prominent in HLA-G negative cells in the proximal column cells (CC, yellow arrow), while pSmad3 (green arrow) was present mostly in nuclei of cells in the distal region where HLA-G positive EVT cells are present (white arrow). Scale bar = 40 mum. STB, syncytiotrophoblast.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3 Active Smad3 is downregulated in preeclamptic placentas. Placentas from PE patients and gestational age-matched controls were assessed for total and phosphorylated (p) Smad2 (A) and Smad3 (B) by Western Blotting. Total Smad2 and Smad3 levels, after normalizing to their respective GAPDH signal, showed no significant difference between the PE and control placentas. The ratio of pSmad2/total Smad2 was also not significantly different between the PE and the corresponding control. However, pSmad3/total Smad3 was significantly decreased (*** p < 0.001) in PE placentas relative to their gestational age-matched controls ( n = 3). The number on the top of each lane indicates gestational age of the sample. PTB, preterm birth; EOPT, early onset preeclampsia; Term, healthy term placentas; LOPE, late onset preeclampsia.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
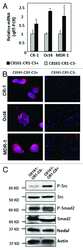
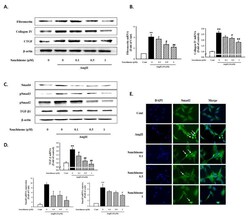
- Figure 3 Effect of sauchinone on AngII-induced mesangial cell fibrosis. ( A ) Western blotting and ( B ) real-time PCR showed protein and mRNA levels of fibronectin, Collagen IV, and CTGF in sauchinone-treated and AngII-stimulated cells at 48 h. ( C , D ) Effect of sauchinone on the relative levels of TGF-beta1/Smads. The results were detected by Western blotting and real-time PCR. ( E ) Immunofluorescent images of p-Smad-2 nuclear translocation under the laser scanning confocal microscopy are show (magnification. 400x). Nuclei were stained with DAPI (blue) and p-smad-2 was stained with Alexa Fluor 488 (green). Green fluorescence indicates localization of p-Smad-2. Respective blot data were obtained from five independent experiments. Each value represents the means +- S.E. of five independent experiments. ** p < 0.01 vs. control and ## p < 0.01, # p < 0.05 vs. AngII alone.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
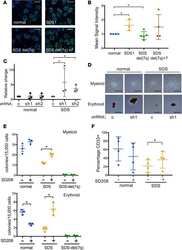
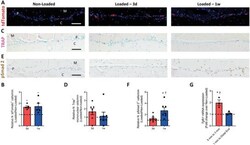
- Myeloid-lineage cells and transforming growth factor- beta 1 (TGF- beta 1) activity were increased in the periosteum with mechanical loading. A-F) Mice underwent 3 d or one week of axial compression loading of the tibiae. Non-loaded tibiae were used as controls. A) Immunofluorescent staining and B) quantification of tdTomato + (red) cells on the periosteal tibial surface in LysM-cre::Ai14 mice. Blue indicates 4',6-diamidino-2-phenylindole (DAPI) staining of nuclei. Scale bar, 50 um. C) Tartrate-resistant acid phosphatase (TRAP) (magenta) staining and D) quantification on periosteal tibial surface in WT mice. Scale bar, 50 um. E) Immunohistochemical staining and F) quantification of pSmad2 + cells (brown) on the periosteal tibial surface in WT mice. Scale bar, 50 um. C, cortical bone; P, periosteum; M, muscle. 3d, 3 days; 1w, one week. G) WT mice underwent one week of axial compression loading of the tibiae. Non-loaded tibiae were used as controls. mRNA expression of Tgfb1 by reverse transcription quantitative polymerase chain reaction (RT-qPCR) extracted from tibial cortical bone. The analyses of (A-F) were performed on the cross sections of posterior and lateral surfaces of tibiae at 5 mm proximal to the distal TFJ. Loaded tibiae values were normalized to the corresponding non-loaded tibiae values. Data are presented as mean +- SEM. n = 5 mice at different time points (B) n = 7 at different time points (D, F) and n = 3 mice (G). B,D,F) * p < 0.05 compared with the correspond
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
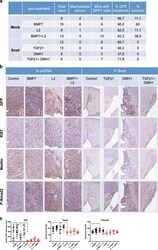
- Fig. 7 Snail suppresses GBM in vivo. a Tumor formation in orthotopically xenografted immunodeficient mice. b Immunohistochemistry of GFP, Ki67, Nestin, and phospho-SMAD2. c Immunohistochemistry quantification: (left) % Ki67-positive nuclei expressed as a percentage of tumor area; (center and right) reciprocal intensity of Nestin and phosho-SMAD2. Data show mean +- SD of 9-20 fields of 3 mice. Statistical comparison (one-way Anova) indicates significant differences at: * p < 0.05, ** p < 0.01, *** p < 0.001
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
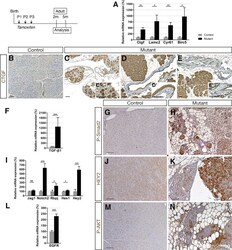
- Figure 6 Ductal deletion of Hnf1b leads to enhanced signaling pathways that favor tumorigenesis. ( A ) RT-qPCR of YAP transcriptional targets. ( B-E ) CTGF (brown) immunohistochemistry. A strong ectopic CTGF staining was observed in ( C' ) acinar cells in mutants in ( D' ) the epithelium of metaplastic ducts and ( E' ) in PanIN. ( F ) RT-qPCR of the Notch pathway. ( G and H ) HEY2 immunohistochemistry. ( I ) RT-qPCR of TGF-beta1 . ( J and K ) Phospho-SMAD2 (Ser465, Ser467) immunohistochemistry. ( L ) RT-qPCR of EGFR . ( M and N ) Phospho-AKT (Ser473) immunohistochemistry. Nuclei were counterstained with hematoxylin. Scale bars : 100 mum. Control, n >= 3; mutant, n >= 3 for immunohistochemistry; and control, n = 6; mutant, n = 5 for RT-qPCR. * P < .05; ** P < .01; *** P < .001.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry