Antibody data
- Antibody Data
- Antigen structure
- References [68]
- Comments [0]
- Validations
- Immunohistochemistry [2]
- Flow cytometry [1]
- Chromatin Immunoprecipitation [2]
- Other assay [34]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 51-1300 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- SMAD2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Pan-Phosphodiesterase Inhibitors Attenuate TGF-β-Induced Pro-Fibrotic Phenotype in Alveolar Epithelial Type II Cells by Downregulating Smad-2 Phosphorylation.
Deletion of Akt1 Promotes Kidney Fibrosis in a Murine Model of Unilateral Ureteral Obstruction.
The neovascularization effect of dedifferentiated fat cells.
A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling.
Heat Shock Protein 90 Involvement in the Development of Idiopathic Epiretinal Membranes.
NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis.
KLF10 Gene Expression Modulates Fibrosis in Dystrophic Skeletal Muscle.
TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation.
MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2.
Acinar-to-Ductal Metaplasia Induced by Transforming Growth Factor Beta Facilitates KRAS(G12D)-driven Pancreatic Tumorigenesis.
Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway.
FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway.
Response to Nodal morphogen gradient is determined by the kinetics of target gene induction.
TGF-β induction of FGF-2 expression in stromal cells requires integrated smad3 and MAPK pathways.
An integrated genomic approach identifies persistent tumor suppressive effects of transforming growth factor-β in human breast cancer.
Brightfield proximity ligation assay reveals both canonical and mixed transforming growth factor-β/bone morphogenetic protein Smad signaling complexes in tissue sections.
Transforming growth factor-β3 (TGF-β3) knock-in ameliorates inflammation due to TGF-β1 deficiency while promoting glucose tolerance.
Cycloheximide inhibits follicle-stimulating hormone β subunit transcription by blocking de novo synthesis of the labile activin type II receptor in gonadotrope cells.
Expression of Wnt and TGF-β pathway components and key adrenal transcription factors in adrenocortical tumors: association to carcinoma aggressiveness.
Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis.
Biological responses to TGF-β in the mammary epithelium show a complex dependency on Smad3 gene dosage with important implications for tumor progression.
Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of β-catenin.
Transforming growth factor β inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes.
Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis.
Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling.
IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling.
Distinctive mechanism for sustained TGF-β signaling and growth inhibition: MEK1 activation-dependent stabilization of type II TGF-β receptors.
Analysis of ligand-dependent nuclear accumulation of Smads in TGF-beta signaling.
Measuring the absolute abundance of the Smad transcription factors using quantitative immunoblotting.
Reduction of transforming growth factor-beta type II receptor is caused by the enhanced ubiquitin-dependent degradation in human renal cell carcinoma.
Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2.
Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V{beta}3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells.
SKI promotes Smad3 linker phosphorylations associated with the tumor-promoting trait of TGFbeta.
Foxa1 functions as a pioneer transcription factor at transposable elements to activate Afp during differentiation of embryonic stem cells.
Induction of ΔNp63 by the newly identified keratinocyte-specific transforming growth factor β Signaling Pathway with Smad2 and IκB Kinase α in squamous cell carcinoma.
Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling.
Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function.
Nox-2 is a modulator of fibrogenesis in kidney allografts.
A positive role for Myc in TGFbeta-induced Snail transcription and epithelial-to-mesenchymal transition.
SKI knockdown inhibits human melanoma tumor growth in vivo.
BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression.
Activins regulate 17beta-hydroxysteroid dehydrogenase type I transcription in murine gonadotrope cells.
Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes.
Smad ubiquitination regulatory factor-2 in the fibrotic kidney: regulation, target specificity, and functional implication.
TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs.
Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells.
Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling.
Generation of mice with conditionally activated transforming growth factor beta signaling through the TbetaRI/ALK5 receptor.
The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties.
Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage.
Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes.
Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney.
Requirement for the dynein light chain km23-1 in a Smad2-dependent transforming growth factor-beta signaling pathway.
LMO4 can interact with Smad proteins and modulate transforming growth factor-beta signaling in epithelial cells.
The mechanism of nuclear export of Smad3 involves exportin 4 and Ran.
Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo.
Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo.
Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells.
Smad7 abrogates transforming growth factor-beta1-mediated growth inhibition in COLO-357 cells through functional inactivation of the retinoblastoma protein.
Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy.
Signaling "cross-talk" between TGF-beta1 and ECM signals in chondrocytic cells.
Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines.
Smad expression in human atherosclerotic lesions: evidence for impaired TGF-beta/Smad signaling in smooth muscle cells of fibrofatty lesions.
BMP and activin receptor expression in lens development.
Involvement of c-Src kinase in the regulation of TGF-beta1-induced apoptosis.
Cellular response to hypoxia involves signaling via Smad proteins.
Reduction in Smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines.
Development and application of fully functional epitope-tagged forms of transforming growth factor-beta.
Wójcik-Pszczoła K, Chłoń-Rzepa G, Jankowska A, Ferreira B, Koczurkiewicz-Adamczyk P, Pękala E, Wyska E, Pociecha K, Gosens R
Pharmaceuticals (Basel, Switzerland) 2022 Mar 30;15(4)
Pharmaceuticals (Basel, Switzerland) 2022 Mar 30;15(4)
Deletion of Akt1 Promotes Kidney Fibrosis in a Murine Model of Unilateral Ureteral Obstruction.
Kim IY, Lee MY, Park MW, Seong EY, Lee DW, Lee SB, Bae SS, Kim SS, Song SH
BioMed research international 2020;2020:6143542
BioMed research international 2020;2020:6143542
The neovascularization effect of dedifferentiated fat cells.
Watanabe H, Goto S, Kato R, Komiyama S, Nagaoka Y, Kazama T, Yamamoto C, Li Y, Konuma N, Hagikura K, Matsumoto T
Scientific reports 2020 Jun 8;10(1):9211
Scientific reports 2020 Jun 8;10(1):9211
A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling.
Wójcik-Pszczoła K, Chłoń-Rzepa G, Jankowska A, Ślusarczyk M, Ferdek PE, Kusiak AA, Świerczek A, Pociecha K, Koczurkiewicz-Adamczyk P, Wyska E, Pękala E, Gosens R
International journal of molecular sciences 2020 Jun 3;21(11)
International journal of molecular sciences 2020 Jun 3;21(11)
Heat Shock Protein 90 Involvement in the Development of Idiopathic Epiretinal Membranes.
Tosi GM, Regoli M, Altera A, Galvagni F, Arcuri C, Bacci T, Elia I, Realini G, Orlandini M, Bertelli E
Investigative ophthalmology & visual science 2020 Jul 1;61(8):34
Investigative ophthalmology & visual science 2020 Jul 1;61(8):34
NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis.
Zhang SZ, Wang QQ, Yang QQ, Gu HY, Yin YQ, Li YD, Hou JC, Chen R, Sun QQ, Sun YF, Hu G, Zhou JW
BMC medicine 2019 Nov 15;17(1):204
BMC medicine 2019 Nov 15;17(1):204
KLF10 Gene Expression Modulates Fibrosis in Dystrophic Skeletal Muscle.
DiMario JX
The American journal of pathology 2018 May;188(5):1263-1275
The American journal of pathology 2018 May;188(5):1263-1275
TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation.
Albers RE, Selesniemi K, Natale DRC, Brown TL
International journal of stem cells 2018 May 30;11(1):111-120
International journal of stem cells 2018 May 30;11(1):111-120
MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2.
Howe GA, Kazda K, Addison CL
PloS one 2017;12(10):e0185619
PloS one 2017;12(10):e0185619
Acinar-to-Ductal Metaplasia Induced by Transforming Growth Factor Beta Facilitates KRAS(G12D)-driven Pancreatic Tumorigenesis.
Chuvin N, Vincent DF, Pommier RM, Alcaraz LB, Gout J, Caligaris C, Yacoub K, Cardot V, Roger E, Kaniewski B, Martel S, Cintas C, Goddard-Léon S, Colombe A, Valantin J, Gadot N, Servoz E, Morton J, Goddard I, Couvelard A, Rebours V, Guillermet J, Sansom OJ, Treilleux I, Valcourt U, Sentis S, Dubus P, Bartholin L
Cellular and molecular gastroenterology and hepatology 2017 Sep;4(2):263-282
Cellular and molecular gastroenterology and hepatology 2017 Sep;4(2):263-282
Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway.
Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK
Endocrinology 2017 Mar 1;158(3):592-603
Endocrinology 2017 Mar 1;158(3):592-603
FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway.
Li J, Shi S, Srivastava SP, Kitada M, Nagai T, Nitta K, Kohno M, Kanasaki K, Koya D
Cell death & disease 2017 Aug 3;8(8):e2965
Cell death & disease 2017 Aug 3;8(8):e2965
Response to Nodal morphogen gradient is determined by the kinetics of target gene induction.
Dubrulle J, Jordan BM, Akhmetova L, Farrell JA, Kim SH, Solnica-Krezel L, Schier AF
eLife 2015 Apr 14;4
eLife 2015 Apr 14;4
TGF-β induction of FGF-2 expression in stromal cells requires integrated smad3 and MAPK pathways.
Strand DW, Liang YY, Yang F, Barron DA, Ressler SJ, Schauer IG, Feng XH, Rowley DR
American journal of clinical and experimental urology 2014;2(3):239-48
American journal of clinical and experimental urology 2014;2(3):239-48
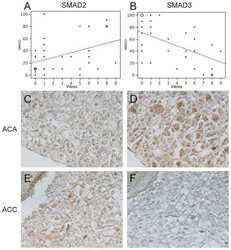
An integrated genomic approach identifies persistent tumor suppressive effects of transforming growth factor-β in human breast cancer.
Sato M, Kadota M, Tang B, Yang HH, Yang YA, Shan M, Weng J, Welsh MA, Flanders KC, Nagano Y, Michalowski AM, Clifford RJ, Lee MP, Wakefield LM
Breast cancer research : BCR 2014 Jun 2;16(3):R57
Breast cancer research : BCR 2014 Jun 2;16(3):R57
Brightfield proximity ligation assay reveals both canonical and mixed transforming growth factor-β/bone morphogenetic protein Smad signaling complexes in tissue sections.
Flanders KC, Heger CD, Conway C, Tang B, Sato M, Dengler SL, Goldsmith PK, Hewitt SM, Wakefield LM
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2014 Dec;62(12):846-63
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2014 Dec;62(12):846-63
Transforming growth factor-β3 (TGF-β3) knock-in ameliorates inflammation due to TGF-β1 deficiency while promoting glucose tolerance.
Hall BE, Wankhade UD, Konkel JE, Cherukuri K, Nagineni CN, Flanders KC, Arany PR, Chen W, Rane SG, Kulkarni AB
The Journal of biological chemistry 2013 Nov 1;288(44):32074-92
The Journal of biological chemistry 2013 Nov 1;288(44):32074-92
Cycloheximide inhibits follicle-stimulating hormone β subunit transcription by blocking de novo synthesis of the labile activin type II receptor in gonadotrope cells.
Rejon CA, Ho CC, Wang Y, Zhou X, Bernard DJ, Hébert TE
Cellular signalling 2013 Jun;25(6):1403-12
Cellular signalling 2013 Jun;25(6):1403-12
Expression of Wnt and TGF-β pathway components and key adrenal transcription factors in adrenocortical tumors: association to carcinoma aggressiveness.
Parviainen H, Schrade A, Kiiveri S, Prunskaite-Hyyryläinen R, Haglund C, Vainio S, Wilson DB, Arola J, Heikinheimo M
Pathology, research and practice 2013 Aug;209(8):503-9
Pathology, research and practice 2013 Aug;209(8):503-9
Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis.
Han G, Bian L, Li F, Cotrim A, Wang D, Lu J, Deng Y, Bird G, Sowers A, Mitchell JB, Gutkind JS, Zhao R, Raben D, ten Dijke P, Refaeli Y, Zhang Q, Wang XJ
Nature medicine 2013 Apr;19(4):421-8
Nature medicine 2013 Apr;19(4):421-8
Biological responses to TGF-β in the mammary epithelium show a complex dependency on Smad3 gene dosage with important implications for tumor progression.
Kohn EA, Yang YA, Du Z, Nagano Y, Van Schyndle CM, Herrmann MA, Heldman M, Chen JQ, Stuelten CH, Flanders KC, Wakefield LM
Molecular cancer research : MCR 2012 Oct;10(10):1389-99
Molecular cancer research : MCR 2012 Oct;10(10):1389-99
Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of β-catenin.
Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, Eschrich SA, Yeatman TJ, Deane NG, Beauchamp RD
Gastroenterology 2012 Mar;142(3):562-571.e2
Gastroenterology 2012 Mar;142(3):562-571.e2
Transforming growth factor β inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes.
Grönroos E, Kingston IJ, Ramachandran A, Randall RA, Vizán P, Hill CS
Molecular and cellular biology 2012 Jul;32(14):2904-16
Molecular and cellular biology 2012 Jul;32(14):2904-16
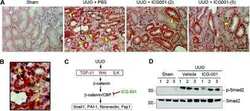
Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis.
Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y
Journal of the American Society of Nephrology : JASN 2011 Sep;22(9):1642-53
Journal of the American Society of Nephrology : JASN 2011 Sep;22(9):1642-53
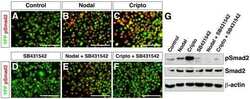
Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling.
Kruithof-de Julio M, Alvarez MJ, Galli A, Chu J, Price SM, Califano A, Shen MM
Development (Cambridge, England) 2011 Sep;138(18):3885-95
Development (Cambridge, England) 2011 Sep;138(18):3885-95
IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling.
Liu Y, Meyer C, Müller A, Herweck F, Li Q, Müllenbach R, Mertens PR, Dooley S, Weng HL
Journal of immunology (Baltimore, Md. : 1950) 2011 Sep 1;187(5):2814-23
Journal of immunology (Baltimore, Md. : 1950) 2011 Sep 1;187(5):2814-23
Distinctive mechanism for sustained TGF-β signaling and growth inhibition: MEK1 activation-dependent stabilization of type II TGF-β receptors.
Chen G, Ghosh P, Longo DL
Molecular cancer research : MCR 2011 Jan;9(1):78-89
Molecular cancer research : MCR 2011 Jan;9(1):78-89
Analysis of ligand-dependent nuclear accumulation of Smads in TGF-beta signaling.
Chapnick DA, Liu X
Methods in molecular biology (Clifton, N.J.) 2010;647:95-111
Methods in molecular biology (Clifton, N.J.) 2010;647:95-111
Measuring the absolute abundance of the Smad transcription factors using quantitative immunoblotting.
Clarke DC, Liu X
Methods in molecular biology (Clifton, N.J.) 2010;647:357-76
Methods in molecular biology (Clifton, N.J.) 2010;647:357-76
Reduction of transforming growth factor-beta type II receptor is caused by the enhanced ubiquitin-dependent degradation in human renal cell carcinoma.
Fukasawa H, Yamamoto T, Fujigaki Y, Misaki T, Ohashi N, Takayama T, Suzuki S, Mugiya S, Oda T, Uchida C, Kitagawa K, Hattori T, Hayashi H, Ozono S, Kitagawa M, Hishida A
International journal of cancer 2010 Oct 1;127(7):1517-25
International journal of cancer 2010 Oct 1;127(7):1517-25
Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2.
Lamba P, Wang Y, Tran S, Ouspenskaia T, Libasci V, Hébert TE, Miller GJ, Bernard DJ
Endocrinology 2010 Nov;151(11):5456-67
Endocrinology 2010 Nov;151(11):5456-67
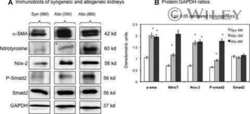
Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V{beta}3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells.
Moyano JV, Greciano PG, Buschmann MM, Koch M, Matlin KS
Molecular biology of the cell 2010 Nov 1;21(21):3654-68
Molecular biology of the cell 2010 Nov 1;21(21):3654-68
SKI promotes Smad3 linker phosphorylations associated with the tumor-promoting trait of TGFbeta.
Lin Q, Chen D, Timchenko NA, Medrano EE
Cell cycle (Georgetown, Tex.) 2010 May;9(9):1684-9
Cell cycle (Georgetown, Tex.) 2010 May;9(9):1684-9
Foxa1 functions as a pioneer transcription factor at transposable elements to activate Afp during differentiation of embryonic stem cells.
Taube JH, Allton K, Duncan SA, Shen L, Barton MC
The Journal of biological chemistry 2010 May 21;285(21):16135-44
The Journal of biological chemistry 2010 May 21;285(21):16135-44
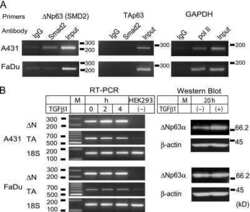
Induction of ΔNp63 by the newly identified keratinocyte-specific transforming growth factor β Signaling Pathway with Smad2 and IκB Kinase α in squamous cell carcinoma.
Fukunishi N, Katoh I, Tomimori Y, Tsukinoki K, Hata R, Nakao A, Ikawa Y, Kurata S
Neoplasia (New York, N.Y.) 2010 Dec;12(12):969-79
Neoplasia (New York, N.Y.) 2010 Dec;12(12):969-79
Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling.
He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y
The Journal of biological chemistry 2010 Aug 6;285(32):24665-75
The Journal of biological chemistry 2010 Aug 6;285(32):24665-75
Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function.
Hall BE, Zheng C, Swaim WD, Cho A, Nagineni CN, Eckhaus MA, Flanders KC, Ambudkar IS, Baum BJ, Kulkarni AB
Laboratory investigation; a journal of technical methods and pathology 2010 Apr;90(4):543-55
Laboratory investigation; a journal of technical methods and pathology 2010 Apr;90(4):543-55
Nox-2 is a modulator of fibrogenesis in kidney allografts.
Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009 Jan;9(1):74-82
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009 Jan;9(1):74-82
A positive role for Myc in TGFbeta-induced Snail transcription and epithelial-to-mesenchymal transition.
Smith AP, Verrecchia A, Fagà G, Doni M, Perna D, Martinato F, Guccione E, Amati B
Oncogene 2009 Jan 22;28(3):422-30
Oncogene 2009 Jan 22;28(3):422-30
SKI knockdown inhibits human melanoma tumor growth in vivo.
Chen D, Lin Q, Box N, Roop D, Ishii S, Matsuzaki K, Fan T, Hornyak TJ, Reed JA, Stavnezer E, Timchenko NA, Medrano EE
Pigment cell & melanoma research 2009 Dec;22(6):761-72
Pigment cell & melanoma research 2009 Dec;22(6):761-72
BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression.
Sehgal R, Sheibani N, Rhodes SJ, Belecky Adams TL
Developmental biology 2009 Aug 15;332(2):429-43
Developmental biology 2009 Aug 15;332(2):429-43
Activins regulate 17beta-hydroxysteroid dehydrogenase type I transcription in murine gonadotrope cells.
Bak B, Carpio L, Kipp JL, Lamba P, Wang Y, Ge RS, Hardy MP, Mayo KE, Bernard DJ
The Journal of endocrinology 2009 Apr;201(1):89-104
The Journal of endocrinology 2009 Apr;201(1):89-104
Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes.
Qureshi HY, Ricci G, Zafarullah M
Biochimica et biophysica acta 2008 Sep;1783(9):1605-12
Biochimica et biophysica acta 2008 Sep;1783(9):1605-12
Smad ubiquitination regulatory factor-2 in the fibrotic kidney: regulation, target specificity, and functional implication.
Tan R, He W, Lin X, Kiss LP, Liu Y
American journal of physiology. Renal physiology 2008 May;294(5):F1076-83
American journal of physiology. Renal physiology 2008 May;294(5):F1076-83
TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs.
Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE
Developmental dynamics : an official publication of the American Association of Anatomists 2008 Jan;237(1):259-69
Developmental dynamics : an official publication of the American Association of Anatomists 2008 Jan;237(1):259-69
Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells.
Flavell JR, Baumforth KR, Wood VH, Davies GL, Wei W, Reynolds GM, Morgan S, Boyce A, Kelly GL, Young LS, Murray PG
Blood 2008 Jan 1;111(1):292-301
Blood 2008 Jan 1;111(1):292-301
Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling.
Le Saux CJ, Teeters K, Miyasato SK, Hoffmann PR, Bollt O, Douet V, Shohet RV, Broide DH, Tam EK
The Journal of biological chemistry 2008 Feb 29;283(9):5760-8
The Journal of biological chemistry 2008 Feb 29;283(9):5760-8
Generation of mice with conditionally activated transforming growth factor beta signaling through the TbetaRI/ALK5 receptor.
Bartholin L, Cyprian FS, Vincent D, Garcia CN, Martel S, Horvat B, Berthet C, Goddard-Léon S, Treilleux I, Rimokh R, Marie JC
Genesis (New York, N.Y. : 2000) 2008 Dec;46(12):724-31
Genesis (New York, N.Y. : 2000) 2008 Dec;46(12):724-31
The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties.
Le Saux O, Teeters K, Miyasato S, Choi J, Nakamatsu G, Richardson JA, Starcher B, Davis EC, Tam EK, Jourdan-Le Saux C
American journal of physiology. Lung cellular and molecular physiology 2008 Dec;295(6):L1007-17
American journal of physiology. Lung cellular and molecular physiology 2008 Dec;295(6):L1007-17
Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage.
Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR
Gastroenterology 2008 Aug;135(2):642-59
Gastroenterology 2008 Aug;135(2):642-59
Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes.
Weng HL, Ciuclan L, Liu Y, Hamzavi J, Godoy P, Gaitantzi H, Kanzler S, Heuchel R, Ueberham U, Gebhardt R, Breitkopf K, Dooley S
Hepatology (Baltimore, Md.) 2007 Oct;46(4):1257-70
Hepatology (Baltimore, Md.) 2007 Oct;46(4):1257-70
Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney.
Banas MC, Parks WT, Hudkins KL, Banas B, Holdren M, Iyoda M, Wietecha TA, Kowalewska J, Liu G, Alpers CE
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2007 Mar;55(3):275-85
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2007 Mar;55(3):275-85
Requirement for the dynein light chain km23-1 in a Smad2-dependent transforming growth factor-beta signaling pathway.
Jin Q, Ding W, Mulder KM
The Journal of biological chemistry 2007 Jun 29;282(26):19122-32
The Journal of biological chemistry 2007 Jun 29;282(26):19122-32
LMO4 can interact with Smad proteins and modulate transforming growth factor-beta signaling in epithelial cells.
Lu Z, Lam KS, Wang N, Xu X, Cortes M, Andersen B
Oncogene 2006 May 11;25(20):2920-30
Oncogene 2006 May 11;25(20):2920-30
The mechanism of nuclear export of Smad3 involves exportin 4 and Ran.
Kurisaki A, Kurisaki K, Kowanetz M, Sugino H, Yoneda Y, Heldin CH, Moustakas A
Molecular and cellular biology 2006 Feb;26(4):1318-32
Molecular and cellular biology 2006 Feb;26(4):1318-32
Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo.
Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB
Journal of immunology (Baltimore, Md. : 1950) 2006 Feb 1;176(3):1316-20
Journal of immunology (Baltimore, Md. : 1950) 2006 Feb 1;176(3):1316-20
Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo.
Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB
Journal of immunology (Baltimore, Md. : 1950) 2006 Feb 1;176(3):1316-20
Journal of immunology (Baltimore, Md. : 1950) 2006 Feb 1;176(3):1316-20
Activin A mediates growth inhibition and cell cycle arrest through Smads in human breast cancer cells.
Burdette JE, Jeruss JS, Kurley SJ, Lee EJ, Woodruff TK
Cancer research 2005 Sep 1;65(17):7968-75
Cancer research 2005 Sep 1;65(17):7968-75
Smad7 abrogates transforming growth factor-beta1-mediated growth inhibition in COLO-357 cells through functional inactivation of the retinoblastoma protein.
Boyer Arnold N, Korc M
The Journal of biological chemistry 2005 Jun 10;280(23):21858-66
The Journal of biological chemistry 2005 Jun 10;280(23):21858-66
Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy.
Romero-Gallo J, Sozmen EG, Chytil A, Russell WE, Whitehead R, Parks WT, Holdren MS, Her MF, Gautam S, Magnuson M, Moses HL, Grady WM
Oncogene 2005 Apr 21;24(18):3028-41
Oncogene 2005 Apr 21;24(18):3028-41
Signaling "cross-talk" between TGF-beta1 and ECM signals in chondrocytic cells.
Schneiderbauer MM, Dutton CM, Scully SP
Cellular signalling 2004 Oct;16(10):1133-40
Cellular signalling 2004 Oct;16(10):1133-40
Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines.
Tian F, Byfield SD, Parks WT, Stuelten CH, Nemani D, Zhang YE, Roberts AB
Cancer research 2004 Jul 1;64(13):4523-30
Cancer research 2004 Jul 1;64(13):4523-30
Smad expression in human atherosclerotic lesions: evidence for impaired TGF-beta/Smad signaling in smooth muscle cells of fibrofatty lesions.
Kalinina N, Agrotis A, Antropova Y, Ilyinskaya O, Smirnov V, Tararak E, Bobik A
Arteriosclerosis, thrombosis, and vascular biology 2004 Aug;24(8):1391-6
Arteriosclerosis, thrombosis, and vascular biology 2004 Aug;24(8):1391-6
BMP and activin receptor expression in lens development.
de Iongh RU, Chen Y, Kokkinos MI, McAvoy JW
Molecular vision 2004 Aug 23;10:566-76
Molecular vision 2004 Aug 23;10:566-76
Involvement of c-Src kinase in the regulation of TGF-beta1-induced apoptosis.
Park SS, Eom YW, Kim EH, Lee JH, Min DS, Kim S, Kim SJ, Choi KS
Oncogene 2004 Aug 19;23(37):6272-81
Oncogene 2004 Aug 19;23(37):6272-81
Cellular response to hypoxia involves signaling via Smad proteins.
Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA
Blood 2003 Mar 15;101(6):2253-60
Blood 2003 Mar 15;101(6):2253-60
Reduction in Smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines.
Tian F, DaCosta Byfield S, Parks WT, Yoo S, Felici A, Tang B, Piek E, Wakefield LM, Roberts AB
Cancer research 2003 Dec 1;63(23):8284-92
Cancer research 2003 Dec 1;63(23):8284-92
Development and application of fully functional epitope-tagged forms of transforming growth factor-beta.
Wolfraim LA, Alkemade GM, Alex B, Sharpe S, Parks WT, Letterio JJ
Journal of immunological methods 2002 Aug 1;266(1-2):7-18
Journal of immunological methods 2002 Aug 1;266(1-2):7-18
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
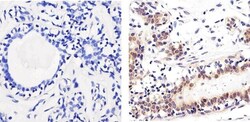
- Experimental details
- Immunohistochemistry analysis of SMAD2 showing staining in the nucleus of paraffin-embedded human breast tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SMAD2 polyclonal antibody (Product # 51-1300) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
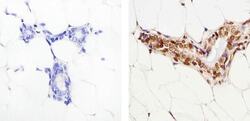
- Experimental details
- Immunohistochemistry analysis of SMAD2 showing staining in the nucleus of paraffin-embedded mouse breast tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SMAD2 polyclonal antibody (Product # 51-1300) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
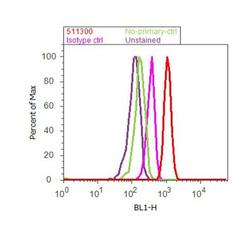
- Experimental details
- Flow cytometry analysis of SMAD2 was done on A549 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with SMAD2 Rabbit Polyclonal Antibody (511300, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
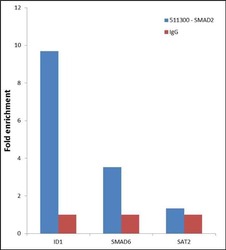
- Experimental details
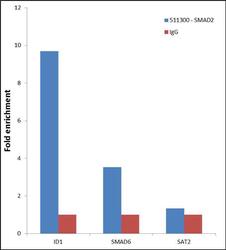
- Enrichment of endogenous SMAD2 Protein at specific gene loci using Anti-SMAD2 Protein Rabbit Polyclonal Antibody: Chromatin Immunoprecipitation (ChIP) was performed using Anti-SMAD2 Protein Rabbit Polyclonal Antibody (Product # 51-1300, 3 µg) on sheared chromatin from 2 million HeLa cells treated with TGF beta (50 ng/mL) for 1 hr using the "MAGnify ChIP system" kit (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by 7500 Fast qPCR system (Product # 4351106) with optimized PCR primer pairs for the promoter of active ID1, SMAD6 gene, used as positive control target, and the SAT2, used as negative control target. Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Enrichment of endogenous SMAD2 Protein at specific gene loci using Anti-SMAD2 Protein Rabbit Polyclonal Antibody: Chromatin Immunoprecipitation (ChIP) was performed using Anti-SMAD2 Protein Rabbit Polyclonal Antibody (Product # 51-1300, 3 µg) on sheared chromatin from 2 million HeLa cells treated with TGF beta (50 ng/mL) for 1 hr using the "MAGnify ChIP system" kit (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by 7500 Fast qPCR system (Product # 4351106) with optimized PCR primer pairs for the promoter of active ID1, SMAD6 gene, used as positive control target, and the SAT2, used as negative control target. Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
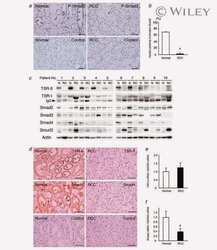
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
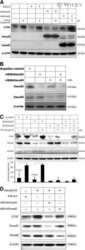
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
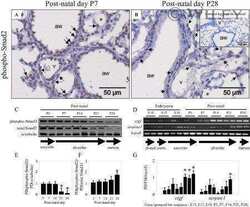
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
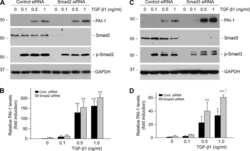
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
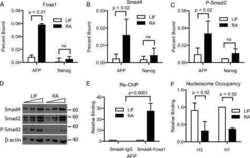
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
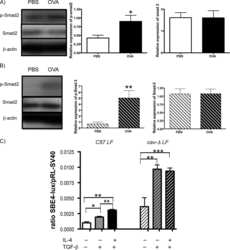
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
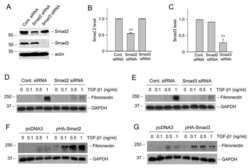
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
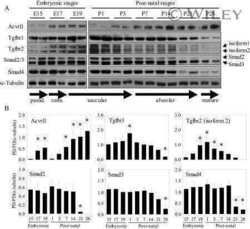
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
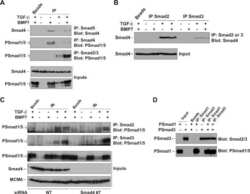
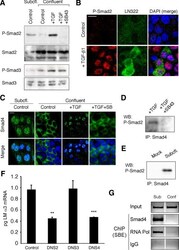
- Figure 4. Phospho-Smad2 and Smad4 regulate LM alpha3 subunit gene transcription. (A) Differential phosphorylation of Smads. Subconfluent (Subcfl.) or confluent cultures either untreated (control) or treated with 5 ng/ml TGF-beta1 for 6 h in the absence or presence of the TbetaR-I inhibitor SB431542 (+TGF-beta1 or + TGF-beta1+SB43, respectively) were analyzed by Western blotting for phospho-Smad2 (P-Smad2), total Smad2, P-Smad3, and total Smad3. (B) Phospho-Smad2 is localized to the nuclei after TGF-beta1 treatment in confluent cells. Untreated (control) or TGF-beta1-treated confluent cells (+TGF-beta1) were stained with antibodies against P-Smad2 (red) and LM-332 (beta3 subunit; green) and analyzed by confocal fluorescence microscopy. Nuclear staining with DAPI, blue. Bar, 10 mum. (C) Smad 4 is also localized to the nuclei in subconfluent and TGF-beta1-treated confluent cells. Subconfluent MDCK cell cultures without added exogenous TGF-beta1 and confluent cultures either untreated (control) or treated with of TGF-beta1 in the presence or absence of SB431542 (SB) were stained with antibodies against Smad4 (green) and analyzed by confocal fluorescence microscopy. Nuclear staining with DAPI, blue. Bar, 10 mum. (D) Phosho-Smad2 and Smad4 form a complex dependent on TbetaR-I signaling. Confluent cultures treated with TGF-beta1 to induce LM-332 expression in the absence or presence of the TbetaR-I inhibitor SB431542 (+TGF or +TGF+SB43, respectively) were extracted in RIPA buffer. E
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
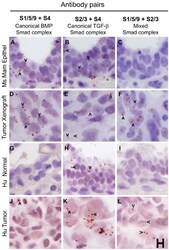
- Figure 6. Mixed Smad complexes are expressed in mammary tumors but not normal mammary epithelium. (A-F) MDA-MB-231 xenograft tumors grown in the mammary fat pads of mice show expression for all three Smad complexes: (D) canonical BMP Smad, (E) canonical TGF-beta Smad and (F) mixed Smad complexes, whereas mammary epithelium, with a more normal histological appearance, shows expression of canonical Smad complexes (A, B), but not mixed Smad complexes (C). (G-L) A human breast tumor shows the expression of canonical and mixed Smads (J-L) whereas normal breast tissue from the same patient expresses the canonical TGF-beta Smad complexes (H) but no mixed Smad complexes (I). Arrowheads mark examples of positive rolling circle amplification (RCA) products in panels with detectable signals. Scale, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
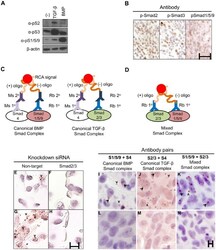
- Figure 3. Proximity ligation assay (PLA) detection of Smad complexes. (A) MDA-MB-231 cells were treated with TGF-beta or BMP2 for 1 hr and pSmad2, pSmad3, or pSmad1/5/9 were detected by western blotting using commercial antibodies. Loading control, beta-actin. (B) Xenograft tumors generated by injection of MDA-MB-231 cells into nude mice were stained with the antibodies used in (A) using peroxidase immunohistochemistry. Scale, 25 um. (C) Canonical Smad complexes were detected by pairing either rabbit (Rb) anti-Smad1/5/9 (left) or anti-Smad2/3 (right) with mouse (Ms) anti-Smad4. The primary (1deg) antibodies are detected by species-specific secondary (2deg) antibodies coupled to either a (+) or (-) oligonucleotide that will hybridize if in close proximity. Following ligation and amplification, the rolling circle amplification (RCA) products are detected by peroxidase-conjugated oligonucleotides and detected with Nova Red. (D) Mixed Smad complexes were detected by conjugating either a (+) or (-) oligonucleotide directly onto the Rb 1deg antibodies and detected as described in (C). (E-H) PLA for mixed Smad complexes in formalin-fixed, paraffin-embedded (FFPE) sections of MDA-MB-231 cells transfected with siRNAs to a non-targeting sequence (E, G) or Smad2/3 (F, H). Cells were treated with TGF-beta (5 ng/ml) for 45 min (G, H) or left untreated (E, F). TGF-beta induced the expression of mixed Smad complexes in cells with non-targeting siRNA (G vs E), but not in cells with Smad2/3 k
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
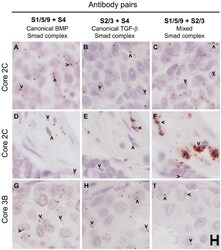
- Figure 7. Human breast cancer tissues from a test tissue microarray (TMA) show different patterns of expression of Smad complexes. The proximity ligation assay was used to detect canonical BMP Smad (A, D, G), canonical TGF-beta Smad (B, E, H) and mixed Smad (C, F, I) complexes in a test TMA of human breast cancer tissues. Images in each row were acquired from similar regions of the tissue cores. A-C and D-F represent two different areas of the same cores, whereas G-I are from an independent core. Arrowheads mark examples of positive rolling circle amplification (RCA) products in panels with detectable signals. Scale, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
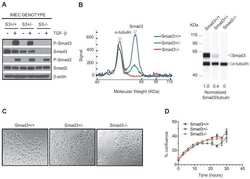
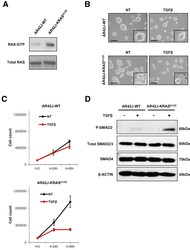
- Figure 1 KRAS G12D potentiates TGFbeta-mediated cell growth inhibition of AR42J rat pancreatic acinar cells. ( A ) Immunoblot of RAS-GTP and total RAS on AR42J-WT and AR42J-KRAS G12D protein extracts. ( B ) Phase microscopy of AR42J-WT and AR42J-KRAS G12D cells 48 hours after TGFbeta treatment. Insets represent higher magnifications fields. Scale bar, 50 mum/L. NT, untreated. *Apoptotic cells. ( C ) Cell count of AR42J-WT and AR42J-KRAS G12D cells at indicated times after TGFbeta treatment. Representative experiment performed in duplicate (means +- standard deviation) is shown. ( D ) Immunoblot of SMAD proteins after 2-hour TGFbeta treatment.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
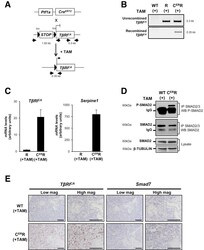
- Figure 6 Inducible TbetaRI CA expression in pancreatic acinar cells after birth results in SMAD pathway activation in vivo . ( A ) Breeding strategy to express TbetaRI CA after birth in pancreatic acinar cells by using tamoxifen-inducible Pft1a-Cre ERT2 allele. Black arrows represent genotyping primers. Sizes of expected fragments are shown. ( B ) PCR on genomic DNA to assess excision of STOP signal in C ER R pancreas after tamoxifen injection. ( C ) RT-qPCR of TbetaRI C A and Serpine-1 on total RNA from pancreata prepared from mice treated with tamoxifen. Expression level in R mice was arbitrarily set at 1 (mean +- standard deviation; 2 independent experiments). ( D ) Immunoprecipitation of SMAD2/3 and Western blot analysis of P-SMAD2 (phospho-SMAD2) and total SMAD2. Total lysate was assessed for beta-tubulin and SMAD2. ( E ) RNAscope detection of TbetaRI CA and Smad7 mRNA on pancreatic sections from WT and C ER R mice. Scale bars, 100 mum. ( A-E ): WT, wild-type; R, [ LSL-TbetaRI CA ]; C ER R, [ Pft1a-Cre ERT2 ; LSL-TbetaRI CA ]. mag, magnification; TAM, tamoxifen.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
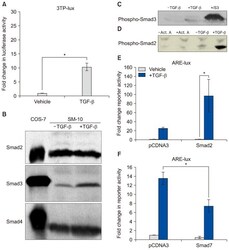
- Fig. 4 TGF- beta triggers Smad2 phosphorylation and transactivation. (A) 3TP luciferase reporter activity in SM10s cells treated with vehicle control or TGF- beta 2. Cells were transiently transfected with 3TP-lux and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle or TGF- beta 2 (5 ng/ml) for 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The 3TP-lux transactivation values were normalized to the constitutively active reporter (pRLSV40). (B) Western blotting analysis of Smad2, Smad3, and Smad4 in TGF- beta 2 or vehicle-treated SM10 cells. COS-7 cells were used as a positive control. (C) Western blotting analysis of Phospho-Smad3 in TGF- beta 2 or vehicle-treated SM10 cells. COS-7 cells transiently transfected with the plasmid, pXIX-Smad3 and treated with TGF- beta were used as a positive control. (D) Western blotting analysis of Phospho-Smad2 in the TGF- beta 2, Activin A, or vehicle-treated SM10 cells. (E) ARE luciferase reporter activity in SM10 cells transiently transfected with pCDNA3 control or pRK5-Smad2 and treated with vehicle control or TGF- beta 2. Cells were transiently transfected with ARE-lux, pLv-CMV-hFAST-1, and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle or TGF- beta 2 (5 ng/ml) for 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The ARE-lux transactivation values were normalized to the const
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
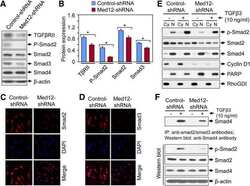
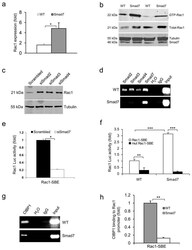
- Figure 3 Smad7 leads to higher Rac1 expression by repressing individual Smad proteins and CtBP1 binding to the SBE of the Rac1 promoter (a ) Rac1 mRNA levels (mean +- s.d.) in keratinocytes from WT and Smad7 transgenic mice. n = 4 per group. ( b ) Western blot analysis of GTP-bound Rac1 (GTP-Rac1) and total Rac1 in WT and Smad7 transgenic keratinocytes. Smad7 protein levels were determined by re-probing the tubulin western blot with an antibody to Smad7 (see an additional western blot and quantification in Supplementary Fig. 4a, b ). (c) The amount of Rac1 protein after knocking down Smad2, Smad3 or Smad4 individually in human keratinocytes (See Supplementary Fig. 4c-e for Smad knockdown efficiencies). siSamd2-4, siRNAs specific for Smad2-4. ( d ) ChIP assay for Smad2, Smad3, Smad4, and Smad7 binding to the SBE -1.5 kb site of the Rac1 promoter in keratinocytes from WT and Smad7 transgenic mice. ( e ) Rac1 luciferase reporter assay in mouse keratinocytes. n = 6 per group. siSmad7, siRNA specific for Smad7; Rac1-SBE, the SBE-1.5 kb site of the Rac1 promoter. Data are the mean +- s.d. ( f ) Activities (mean +- s.d.) of Rac1- luc reporters containing SBE (Rac1-SBE) or mutant SBE (Mut Rac1-SBE) in keratinocytes from WT and Smad7 transgenic mice. n = 6 per group. ( g ) Images of ChIP assays of CtBP1 binding to the SBE-1.5 kb site of the Rac1 promoter in keratinocytes from WT or K5.Smad7 mice. ( h ) ChIP-quantitative PCR (mean +- s.d.) of CtBP1 binding to the SBE in g in keratinocy
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
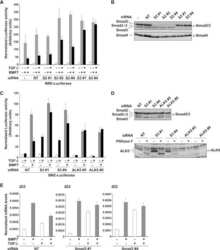
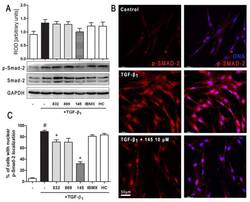
- Figure 6 Compound 145 limits TGF-beta 1 -induced Smad-2 phosphorylation and nuclear translocation in MRC-5 cells. Lung fibroblasts were pre-incubated with the studied compounds (10 uM) for 1 h, and then TGF-beta 1 (5 ng/mL) was added for 1 h. ( A ) Representative immunoblot and densitometry analysis of p-Smad-2 in MRC-5 ( n = 4). The relative optical density (ROD) signal was normalized to the GAPDH control level. Each bar represents the mean value (+- S.E.M.). ( B ) Immunofluorescence staining for p-Smad-2 protein: MRC-5 were fixed, permeabilized, blocked, and incubated with anti-p-Smad-2 antibody followed by Alexa Fluor 546 conjugated antibody, nuclei were stained with the Hoechst 33342 dye. Microphotographs were taken using Leica DMi8 microscope with Thunder Imaging System, 40x objective, bar = 50 um. ( C ) Cells exhibiting strong nuclear p-Smad-2 signals were counted in 20 randomly selected fields of view and expressed as a percentage fraction in the entire MRC-5 population. The results were considered statistically significant at the p level of 0.05, against the control (#) and TGF-beta 1 (*) conditions.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
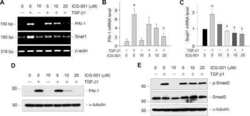
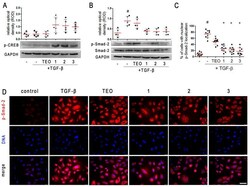
- Pan-PDE inhibitors attenuate TGF-beta-induced Smad-2 signaling in alveolar epithelial type II cells. A549 cells were cultured overnight and then serum-deprived for 24 h. 1 , 2 , 3 , or TEO (10 muM) was administrated 1 h before TGF-beta (5 ng/mL) treatment for an additional 1 h incubation. Representative immunoblots and densitometry analysis of p-CREB ( A ) and p-Smad-2 ( B ) in A549 ( n = 5). The relative optical density was normalized to the GAPDH control level. Horizontal lines represent the median with interquartile range (Kruskal-Wallis test with Dunn's post-hoc test). ( D ) Representative images (400x magnification) of A549 immunostained for p-Smad-2. Cells were fixed, permeabilized, blocked, and incubated with anti-p-Smad-2 antibody followed by an Alexa Fluor 546 conjugated antibody, and nuclei were co-stained with Hoechst 33342 dye. Scale bar = 50 um. ( C ) Cells exhibiting colocalization of p-Samd-2 and nuclear signal were counted in randomly selected fields of view and expressed as a percentage fraction in the entire A549 population. Horizontal lines represent the mean value (+-SD), n = 10 (ANOVA with Dunnett's post-hoc test). The results were considered statistically significant at the p -level of 0.05, against the control (#) and TGF-beta (*) conditions.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
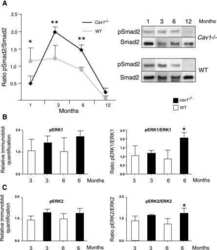
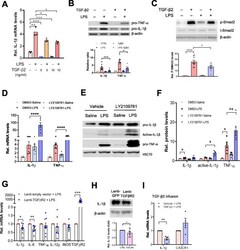
- Fig. 6 TGF-ss2/TGFBR2 signaling regulates microglial homeostasis via CX3CR1. a Immunofluorescent staining of representative sections showing TGF-beta2 and NG2 immunoreactivities in the corpus callosum of wild-type mice. Arrows indicate double-labeled cells. Scale bar, 50 mum. b Measurement of TGF-beta2 protein levels was performed in the concentrated conditioned medium (CM) from primary NG2 glial cells using TGF-beta2 ELISA ( n = 6 for control medium, n = 7 for NG2 glia CM). Unpaired t test. ** P < 0.01. c Pre-incubation of NG2 CM with antibody against TGF-ss2 markedly reduces the mRNA levels of CX3CR1 and increases IL-1beta expression in primary cultured microglia. Unpaired two-tailed t test, ( n = 8-10). *** P < 0.001 for CX3CR1. Nonparametric test with Mann-Whitney test, ** P < 0.01 for IL-1beta. All data are expressed as mean +- SEM. d qPCR analysis shows that the increases in the expression of Tmem119 and CX3CR1 in microglial cell line BV2 cells were abolished upon addition of LY2109761, a TGFBR1/2 blocker. One-way ANOVA with Newman-Keul's post test (6-8 independent experiments). * P < 0.05, ** P < 0.01, **** P < 0.001. e Immunoblotting analysis reveals that the elevations in the levels of phopho-Smad2 evoked by TGF-beta2 are blocked by LY2109761 in BV2 cells. One-way ANOVA with Newman-Keul's post test ( n = 3 independent experiments). **** P < 0.001. f qPCR analysis shows that the decreases in the expression of CX3CR1 and Tmem119 in BV2 cells were induced upon transfect
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 7 Activation of TGF-beta2/TGFBR2 signaling attenuates LPS-induced neuroinflammation. a qPCR analysis showing the reduction of IL-1beta mRNA levels in primary cultured microglia treated with TGF-beta2 (5, 10 ng ml -1 , 4 h) in the presence or absence of LPS (50 ng ml -1 ). Unpaired t test ( n = 3 independent experiments). b , c Representative immunoblots showing the increases in the levels of IL-1beta and TNF-a protein induced by LPS were attenuated by TGF-beta2 treatment in BV2 cells ( n = 8 independent experiments). This process is accompanied by alterations in the levels of phospho-SMAD2 ( c ). n = 9 independent experiments. d qPCR analysis shows that the disruption of TGFBR2 signaling by LY2109761 exacerbates inflammatory response to LPS stimulation. BV2 cells were treated with LY2109761 (10 ng/ml) or vehicle DMSO for 2 h, followed by exposure to LPS (100 ng/ml) or saline for 6 h. Two-way ANOVA with Turkey's test ( n = 6 independent experiments). e , f Immunoblot analysis showing the increases in the levels of active-IL-1beta in BV2 cells with disruption of TGFBR2 signaling by LY2109761. f Quantification of data shown in e . Two-way ANOVA with Turkey's test. Data are expressed as mean +- SEM ( n = 3-6). * P < 0.05, ** P < 0.01, *** P < 0.001. g qPCR analysis of the striatum of wild-type mice that received stereotactic injection of lentivirus-TGFBR2 showed that the production of inflammatory mediators was attenuated by TGFbetaR2 overexpression
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
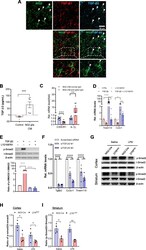
- Figure 5--figure supplement 1. Characterization of direct Nodal target genes. Left panel: binding peaks of Smad2 and its associated transcription factor FoxH1 at dome stage after injection of zebrafish Nodal Squint mRNA (red) or after treatment with the Nodal signaling inhibitor SB505124 (blue). Peaks called by the MACS algorithm are indicated (gray blocks). Middle panel: NanoString count levels after injection of recombinant mouse Nodal protein (red) or after SB505124 treatment (blue) in the absence (-cycloH) or in the presence (+cycloH) of the translation inhibitor cycloheximide. Right panel: time course induction of target genes after Nodal injection at 3.5 hpf (green) or 4.5 hpf (orange). In each case, (1) a specific Smad2 peak associated with a FoxH1 peak appears in the vicinity of the TSS upon Squint injection, (2) the Nodal-induced expression is not abolished by cycloheximide treatment, and (3) induction kinetics have similar trajectories independently of the stage of Nodal exposure, suggesting that these genes are direct targets of Nodal signaling. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 8--figure supplement 1. Characterization of co-regulated Nodal target genes. Left panel: binding peaks of Smad2 and the associated transcription factor FoxH1 at dome stage after injection of Squint mRNA (red) or after treatment with the Nodal signaling inhibitor SB505124 (blue). Peaks called by the MACS algorithm are indicated (gray blocks). Middle panel: NanoString count levels after mNodal injection (red) or after SB treatment (blue) in the absence (-cycloH) or the presence (+cycloH) of the translation inhibitor cycloheximide. Right panel: time course induction of target genes after Nodal injection at 3.5 hpf (green) or 4.5 hpf (orange). Although these genes have Smad2/FoxH1 binding sites, their induction is abolished in the presence of cycloheximide, suggesting that an additional transcriptional co-regulator controls their transcription. Moreover, these genes are delayed in their onset of expression. The length of the delay depends on the stage at which Nodal is applied. DOI: http://dx.doi.org/
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunohistochemistry
Immunohistochemistry