Antibody data
- Antibody Data
- Antigen structure
- References [28]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [13]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 17-0389-41 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD38 Monoclonal Antibody (HIT2), APC, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The HIT2 monoclonal antibody reacts with the human CD38 molecule, an approximately 45 kDa type II transmembrane protein. CD38 is expressed by thymocytes, peripheral B cells including plasma cells, activated T cells, and monocytes. CD38 is a counter-receptor for CD31 and has both cyclase and hydrolase enzymatic activity. Applications Reported: This HIT2 antibody has been reported for use in flow cytometric analysis. Applications Tested: This HIT2 antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (0.25 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 633-647 nm; Emission: 660 nm; Laser: Red Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- HIT2
- Vial size
- 25 Tests
- Concentration
- 5 µL/Test
- Storage
- 4°C, store in dark, DO NOT FREEZE!
Submitted references Novel myeloma patient-derived xenograft models unveil the potency of anlotinib to overcome bortezomib resistance.
B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction.
Enhanced CXCR4 Expression of Human CD8(Low) T Lymphocytes Is Driven by S1P(4).
Genetic manipulation and immortalized culture of ex vivo primary human germinal center B cells.
SARS-CoV-2 infection paralyzes cytotoxic and metabolic functions of the immune cells.
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen dysregulates expression of MCL-1 by targeting FBW7.
Highly efficient CRISPR-Cas9-mediated gene knockout in primary human B cells for functional genetic studies of Epstein-Barr virus infection.
Patient iPSC-Derived Macrophages to Study Inborn Errors of the IFN-γ Responsive Pathway.
IFNγ induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation.
CD16(+)NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice.
Genetic Architecture of Adaptive Immune System Identifies Key Immune Regulators.
A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade.
Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial.
Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells.
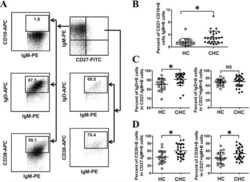
Abnormal phenotypic features of IgM+B cell subsets in patients with chronic hepatitis C virus infection.
Renal Transplant Recipients Treated with Calcineurin-Inhibitors Lack Circulating Immature Transitional CD19+CD24hiCD38hi Regulatory B-Lymphocytes.
PD-L1 mediated the differentiation of tumor-infiltrating CD19(+) B lymphocytes and T cells in Invasive breast cancer.
SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment.
Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells.
Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling.
DNA methylation profiling in human B cells reveals immune regulatory elements and epigenetic plasticity at Alu elements during B-cell activation.
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Further phenotypic characterization of the primitive lineage- CD34+CD38-CD90+CD45RA- hematopoietic stem cell/progenitor cell sub-population isolated from cord blood, mobilized peripheral blood and patients with chronic myelogenous leukemia.
Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers.
Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells.
CD38 binding to human myeloid cells is mediated by mouse and human CD31.
Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member.
The labelling index of primitive plasma cells determines the clinical behaviour of patients with myelomatosis.
Yue Y, Cao Y, Mao X, Wang F, Fan P, Qian L, Guo S, Li F, Guo Y, Chen T, Lin Y, Dong W, Liu Y, Huang Y, Gu W
Frontiers in oncology 2022;12:894279
Frontiers in oncology 2022;12:894279
B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction.
Lee JL, Fra-Bido SC, Burton AR, Innocentin S, Hill DL, Linterman MA
Aging cell 2022 Sep;21(9):e13692
Aging cell 2022 Sep;21(9):e13692
Enhanced CXCR4 Expression of Human CD8(Low) T Lymphocytes Is Driven by S1P(4).
Burkard T, Dreis C, Herrero San Juan M, Huhn M, Weigert A, Pfeilschifter JM, Radeke HH
Frontiers in immunology 2021;12:668884
Frontiers in immunology 2021;12:668884
Genetic manipulation and immortalized culture of ex vivo primary human germinal center B cells.
Caeser R, Gao J, Di Re M, Gong C, Hodson DJ
Nature protocols 2021 May;16(5):2499-2519
Nature protocols 2021 May;16(5):2499-2519
SARS-CoV-2 infection paralyzes cytotoxic and metabolic functions of the immune cells.
Singh Y, Trautwein C, Fendel R, Krickeberg N, Berezhnoy G, Bissinger R, Ossowski S, Salker MS, Casadei N, Riess O, Deutsche COVID-19 OMICS Initiate (DeCOI)
Heliyon 2021 Jun;7(6):e07147
Heliyon 2021 Jun;7(6):e07147
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen dysregulates expression of MCL-1 by targeting FBW7.
Kim YJ, Kim Y, Kumar A, Kim CW, Toth Z, Cho NH, Lee HR
PLoS pathogens 2021 Jan;17(1):e1009179
PLoS pathogens 2021 Jan;17(1):e1009179
Highly efficient CRISPR-Cas9-mediated gene knockout in primary human B cells for functional genetic studies of Epstein-Barr virus infection.
Akidil E, Albanese M, Buschle A, Ruhle A, Pich D, Keppler OT, Hammerschmidt W
PLoS pathogens 2021 Apr;17(4):e1009117
PLoS pathogens 2021 Apr;17(4):e1009117
Patient iPSC-Derived Macrophages to Study Inborn Errors of the IFN-γ Responsive Pathway.
Haake K, Neehus AL, Buchegger T, Kühnel MP, Blank P, Philipp F, Oleaga-Quintas C, Schulz A, Grimley M, Goethe R, Jonigk D, Kalinke U, Boisson-Dupuis S, Casanova JL, Bustamante J, Lachmann N
Cells 2020 Feb 19;9(2)
Cells 2020 Feb 19;9(2)
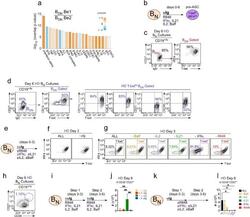
IFNγ induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation.
Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B, Rosal-Vela A, Botta D, Bradley JE, Wojciechowski W, Ptacek T, Danila MI, Edberg JC, Bridges SL Jr, Kimberly RP, Chatham WW, Schoeb TR, Rosenberg AF, Boss JM, Sanz I, Lund FE
eLife 2019 May 15;8
eLife 2019 May 15;8
CD16(+)NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice.
Williams BA, Wang XH, Leyton JV, Maghera S, Deif B, Reilly RM, Minden MD, Keating A
Haematologica 2018 Oct;103(10):1720-1729
Haematologica 2018 Oct;103(10):1720-1729
Genetic Architecture of Adaptive Immune System Identifies Key Immune Regulators.
Lagou V, Garcia-Perez JE, Smets I, Van Horebeek L, Vandebergh M, Chen L, Mallants K, Prezzemolo T, Hilven K, Humblet-Baron S, Moisse M, Van Damme P, Boeckxstaens G, Bowness P, Dubois B, Dooley J, Liston A, Goris A
Cell reports 2018 Oct 16;25(3):798-810.e6
Cell reports 2018 Oct 16;25(3):798-810.e6
A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade.
Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, Savic Prince S, Wiese M, Lardinois D, Ho PC, Klein C, Karanikas V, Mertz KD, Schumacher TN, Zippelius A
Nature medicine 2018 Jul;24(7):994-1004
Nature medicine 2018 Jul;24(7):994-1004
Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial.
Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA, Weiss ST, Carey VJ, O'Connor G, Hawrylowicz C
The Journal of allergy and clinical immunology 2018 Jan;141(1):269-278.e1
The Journal of allergy and clinical immunology 2018 Jan;141(1):269-278.e1
Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells.
Fujishiro A, Miura Y, Iwasa M, Fujii S, Sugino N, Andoh A, Hirai H, Maekawa T, Ichinohe T
Inflammation and regeneration 2017;37:19
Inflammation and regeneration 2017;37:19
Abnormal phenotypic features of IgM+B cell subsets in patients with chronic hepatitis C virus infection.
Kong F, Feng B, Zhang H, Rao H, Wang J, Cong X, Wei L
Experimental and therapeutic medicine 2017 Aug;14(2):1846-1852
Experimental and therapeutic medicine 2017 Aug;14(2):1846-1852
Renal Transplant Recipients Treated with Calcineurin-Inhibitors Lack Circulating Immature Transitional CD19+CD24hiCD38hi Regulatory B-Lymphocytes.
Tebbe B, Wilde B, Ye Z, Wang J, Wang X, Jian F, Dolff S, Schedlowski M, Hoyer PF, Kribben A, Witzke O, Hoerning A
PloS one 2016;11(4):e0153170
PloS one 2016;11(4):e0153170
PD-L1 mediated the differentiation of tumor-infiltrating CD19(+) B lymphocytes and T cells in Invasive breast cancer.
Guan H, Lan Y, Wan Y, Wang Q, Wang C, Xu L, Chen Y, Liu W, Zhang X, Li Y, Gu Y, Wang Z, Xie F
Oncoimmunology 2016 Feb;5(2):e1075112
Oncoimmunology 2016 Feb;5(2):e1075112
SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment.
Mian SA, Rouault-Pierre K, Smith AE, Seidl T, Pizzitola I, Kizilors A, Kulasekararaj AG, Bonnet D, Mufti GJ
Nature communications 2015 Dec 8;6:10004
Nature communications 2015 Dec 8;6:10004
Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells.
Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, Hewitt E, Salama AD, Baker RJ
Journal of the American Society of Nephrology : JASN 2014 Jul;25(7):1575-85
Journal of the American Society of Nephrology : JASN 2014 Jul;25(7):1575-85
Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling.
Fernandez I, Ooi TP, Roy K
Stem cells (Dayton, Ohio) 2014 Jan;32(1):93-104
Stem cells (Dayton, Ohio) 2014 Jan;32(1):93-104
DNA methylation profiling in human B cells reveals immune regulatory elements and epigenetic plasticity at Alu elements during B-cell activation.
Lai AY, Mav D, Shah R, Grimm SA, Phadke D, Hatzi K, Melnick A, Geigerman C, Sobol SE, Jaye DL, Wade PA
Genome research 2013 Dec;23(12):2030-41
Genome research 2013 Dec;23(12):2030-41
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Radomska HS, Alberich-Jordà M, Will B, Gonzalez D, Delwel R, Tenen DG
The Journal of clinical investigation 2012 Aug;122(8):2955-66
The Journal of clinical investigation 2012 Aug;122(8):2955-66
Further phenotypic characterization of the primitive lineage- CD34+CD38-CD90+CD45RA- hematopoietic stem cell/progenitor cell sub-population isolated from cord blood, mobilized peripheral blood and patients with chronic myelogenous leukemia.
Wisniewski D, Affer M, Willshire J, Clarkson B
Blood cancer journal 2011 Sep;1(9):e36
Blood cancer journal 2011 Sep;1(9):e36
Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers.
Bentebibel SE, Schmitt N, Banchereau J, Ueno H
Proceedings of the National Academy of Sciences of the United States of America 2011 Aug 16;108(33):E488-97
Proceedings of the National Academy of Sciences of the United States of America 2011 Aug 16;108(33):E488-97
Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells.
Nagata S, Ise T, Pastan I
Journal of immunology (Baltimore, Md. : 1950) 2009 Jun 15;182(12):7518-26
Journal of immunology (Baltimore, Md. : 1950) 2009 Jun 15;182(12):7518-26
CD38 binding to human myeloid cells is mediated by mouse and human CD31.
Horenstein AL, Stockinger H, Imhof BA, Malavasi F
The Biochemical journal 1998 Mar 15;330 ( Pt 3):1129-35
The Biochemical journal 1998 Mar 15;330 ( Pt 3):1129-35
Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member.
Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F
Journal of immunology (Baltimore, Md. : 1950) 1998 Jan 1;160(1):395-402
Journal of immunology (Baltimore, Md. : 1950) 1998 Jan 1;160(1):395-402
The labelling index of primitive plasma cells determines the clinical behaviour of patients with myelomatosis.
Joshua D, Petersen A, Brown R, Pope B, Snowdon L, Gibson J
British journal of haematology 1996 Jul;94(1):76-81
British journal of haematology 1996 Jul;94(1):76-81
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
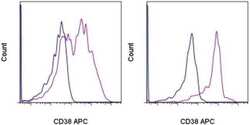
- Experimental details
- Staining of normal human peripheral blood cells with Mouse IgG1 K Isotype Control APC (Product # 17-4714-81) (blue histogram) or Anti-Human CD38 APC (purple histogram). Cells in the lymphocyte gate (left) and monocyte gate (right) were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
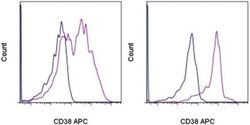
- Experimental details
- Staining of normal human peripheral blood cells with Mouse IgG1 K Isotype Control APC (Product # 17-4714-81) (blue histogram) or Anti-Human CD38 APC (purple histogram). Cells in the lymphocyte gate (left) and monocyte gate (right) were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
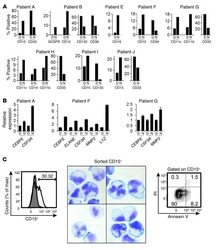
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4. IFNgamma is required for development of T-bet hi B DN cells and regulates ASC formation and recovery. ( a ) Ingenuity Pathway Analysis (IPA) to identify predicted upstream direct and indirect regulators of the HD B DN Be1 transcriptome. IPA performed using the 427 DEG (B DN Be1 over B DN Be2; FDR < 0.05) identified in the RNA-seq analysis described in Figure 3b . The predicted activation state (z-score of B DN Be1 over B DN Be2) of each regulator/signaling pathway is shown as bar color (orange, activated; blue, inhibited) with predicted upstream regulators sorted in order of significance (overlap P value). Regulators with an overlap P -value
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
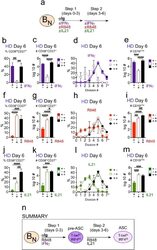
- Figure 5. Temporally distinct regulation of T-bet hi IRF4 int pre-ASC and ASC development by IFNgamma, R848 and IL-21. Cartoon ( a ) depicting stimulation of CTV-labeled HD B N cells for 3 days with anti-Ig, R848, IL-21 and IFNgamma (Step 1). Cells were washed and re-cultured for 3 days with R848, IFNgamma, and IL-21 (Step 2, +,+ condition) or individual stimuli were included in Step 1 only (+,- condition) or in Step 2 only (-,+ condition). Cells from day 6 cultures containing IFNgamma ( b-e ), R848 ( f-i ) or IL-21 ( j-m ) in Step 1, Step 2 or both steps were analyzed to determine ASC frequencies ( b, f, j ), ASC recovery ( c, g, k ), cell division ( d, h, l ) and total cell recovery ( e, i, m ). Summary of data ( n ) showing that ASC development and recovery from T-bet hi IRF4 int B DN pre-ASCs requires early IFNgamma, R848 and BCR ''priming'' signals and late R848 and IL-21 proliferation and differentiation signals. See Figure 5-figure supplement 1 for representative flow cytometry plots from each culture showing T-bet hi IRF4 int B DN cells on day 3, CD38 hi CD27 + ASCs on day 6 and CTV dilution on day 6. Data are representative of >=3 experiments. The percentage of cells in each division, the frequency of ASCs and cell recovery (total and ASCs) are shown as the mean +-SD of cultures containing purified B N cells from 3 independent healthy donors. All statistical analyses were performed using one-way ANOVA with Tukey''s multiple comparison test. P values *
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
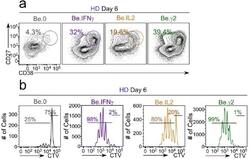
- Figure 6-figure supplement 2. Flow cytometric characterization of B cells activated during the early priming phase in the presence or absence of IFNgamma and IL-2. Day 6 Be.0, Be.IL2, Be.IFNgamma and Be.gamma2 cells were generated as described in Figure 6e . Representative flow cytometry plots from day 6 Be.0, Be.IFNgamma, Be.IL2 and Be.gamma2 cultures showing CD38 hi CD27 + ASCs ( a ) and CTV dilution ( b ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6. IFNgamma cooperates with R848, IL-2 and IL-21 to promote development and recovery of ASCs. ( a-d ) IFNgamma synergizes with subthreshold amounts of TLR7/8 ligand to induce proliferation and differentiation of B N cells. CTV-labeled HD B N cells were activated for 3 days (Step 1) with anti-Ig, IL-2, and increasing concentrations of R848 (as indicated) in the presence or absence of IFNgamma (10 ng/ml). Cells were washed and re-cultured for 3 additional days (Step 2) with IL-21 and the same concentration of R848 that was used in Step 1. B cell division was measured on day 6 in cultures that were activated with IFNgamma (green circles) or without IFNgamma (orange circles) in the presence of no R848 (0 mug/ml, ( a ), high dose R848 (10 mug/ml, ( b ) or low dose R848 (0.1 mug/ml, ( c ). The frequency of CD38 hi CD27 + ASCs ( d ) on day 6 is shown. ( e-i ) IFNgamma cooperates with IL-2 to promote ASC development and recovery. Cartoon ( e ) depicting CTV-labeled HD B N cells activated for 3 days (Step 1) with anti-Ig and R848 alone (Be.0); with anti-Ig +R848+IFNgamma (Be.IFNgamma); with anti-Ig +R848+IL-2 (Be.IL2); or with anti-Ig +R848+IFNgamma+IL-2 (Be.gamma2). Cells were then washed and recultured for an additional 3 days (Step 2) with R848 and IL-21. The percentage of cells that have undergone cell division ( f ), the total cell recovery ( g ), the ASC frequencies ( h ) and total ASCs recovered ( i ) from each day 6 culture are shown. ( j-k ) Early IFNgamma signals regu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
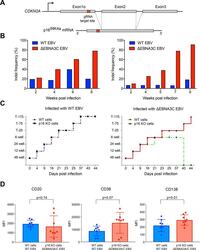
- Fig 4 p16 INK4a is a functional barrier to EBV driven proliferation of lymphoblastoid cells. (A) Blueprint of the primary transcript and the spliced mRNA with the three exons of CDKN2A on chromosome 9 encoding the p16 INK4a protein. The target site of the RNP complex within the 1st exon (exon1alpha) (chr9:21,974,678-21,974,827) is shown. (B) Study of the biological effect of the CDKN2A knockout in a time course experiment. WT and p16 KO cells were mixed such that the fraction of the latter was in the order of 10 to 20%, when the cells were infected with WT or DeltaEBNA3C EBV strains. The knockout status of the CDKN2A gene was studied by next generation sequencing to analyze the CD46 locus of the mixed cell populations over time. The fraction of cells with a disabled CDKN2A gene increased in cells infected with DeltaEBNA3C EBV exceeding 80% after eight weeks, whereas the knockout status of CDKN2A in the population of cells infected with WT EBV did not show a clear trend. Results from two biological replicates are shown, additional replicates can be found in S4A Fig . (C) Cell numbers of four different B cell populations were plotted as a function of days post nucleofection (x-axis) versus the format of the cell culture vessel (y-axis) starting with a single well in a 48-well cluster plate. 2x10 6 B cells with an intact CDKN2A locus (WT cells) or cells with an edited CDKN2A gene (p16 KO cells) were infected with wild-type (WT) EBV (left panel) or DeltaEBNA3C EBV (right panel).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
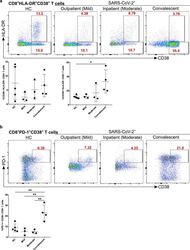
- Figure 7 Increased exhausted CD8 + T cells in convalescent patients. A. Expression of activation marker CD38 on CD8 + T cells (upper FACS panel). One exemplary dot plot is shown per study group. The bar diagram (lower panel) shows that CD38 expression was significantly higher on CD8 + T cells in convalescent COVID-19 + patients compared with HC. *P-value
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
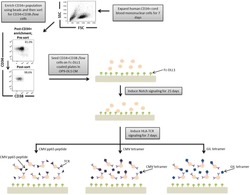
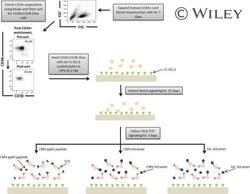
- A schematic overview of our experimental design to generate antigen-specific CD8+ T cells. CD34+ CB cells were expanded for 7 days and CD34+CD38-/low cells were isolated. After 7 days of CD34+ cell expansion, CD34+ cells were enriched using magnetic bead separation. The enriched cells were stained with anti-CD34 and anti-CD38 antibodies to isolate the CD34+CD38-/low HSC population. 81.0% of FSC versus SSC gated cells were CD34+CD38-/low. Gate was determined by the isotype staining control. Data are representative of at least six independent experiments. To induce Notch signaling and early T cell differentiation, CD34+CD38-/low cells were seeded onto DLL1-coated nontissue culture-treated plates. HLA-TCR signaling to direct antigen-specific differentiation was induced using CMVpp65 peptide or epitope-loaded HLA-A*0201 class I tetramers or GIL epitope-loaded HLA-A*0201 class I tetramers. Abbreviations: CM, conditioned medium; CMV, cytomegalovirus; FSC, Forward Scatter; GIL, Influenza M1 virus; HLA, Human leukocyte antigen; SSC, Side Scatter; TCR, T cell receptor.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
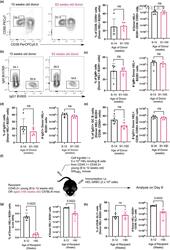
- 7 FIGURE B cells from aged donor mice do not have defects in participating in the GC 10 days after immunization in young recipient mice and fewer B cells from young donor mice are recovered when transferred into aged recipient mice. (a) Representative flow cytometric plots of CD38 - CD95 + GC B cells gated on donor cells from 10-week-old or 93-week-old donor mice in recipient spleens on day 10 post-transfer and immunisation. Numbers adjacent to gates indicate percentage of donor HEL + B220 + cells. Percentage and number of donor-derived GC B cells (Donor HEL + B220 + CD38 - CD95 + ) are plotted on the graphs on the right. (b) Representative flow cytometric plots of IgM + and IgG1 + donor HEL + B cells. Numbers adjacent to gates indicate percentage of donor HEL + B220 + cells. (c, d) Percentage and number of (c) IgM + and (d) IgG1 + B cells derived from donor cells from young or aged mice in recipient spleens 10 days post-transfer and immunisation. (e) Graph showing the percentage and number of IgG1 + GC B cells out of total Donor HEL + B220 + CD38 - CD95 + cells. (f) Schematic diagram of adoptive transfer of B cells from young SW HEL mice into young (8-12 weeks old) or aged (>90 weeks old) mice in which B cells response was analysed 6 days post-transfer and immunisation. (g) Percentage and number of donor HEL + B220 + cells in spleens of young or aged recipient mice 6 days post-transfer and immunisation. (h) Percentage and number of donor-derived GC B cells (Donor HEL + B220
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
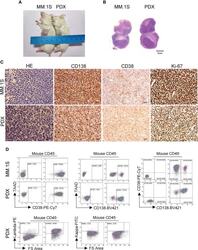
- Morphological and phenotypic features of tumor cells from newly established myeloma PDX. MM.1S cells and mononuclear cells isolated from BM and pleural effusion samples of MM patient were inoculated subcutaneously in NDG mice. When tumors reached 6-8 mm in diameter, tumors were removed and prepared for HE staining, IHC staining, and single-cell suspensions. (A) Representative image of myeloma MM.1S and PDX models. (B) The morphology of tumor cells from myeloma MM.1S and PDX models by Giemsa staining. Scale bar: 5 mum. (C) Representative IHC stainings of tumor tissues. Scale bar: 100 mum. (D) The single-cell suspensions were analyzed by flow cytometry. The doublet or aggregated events were gated out according to side scatter area (SSC-A) and side scatter width (SSC-W). 7-AAD staining was used to gate out dead cells. The expression levels of CD138, CD38, kappa, and lambda were analyzed in mouse CD45 - cells. Myeloma cell line MM.1S was used as a positive myeloma cell control. The left panels of flow charts were isotype controls. PDX, patient-derived xenograft; HE, hematoxylin-eosin; IHC, immunohistochemical.
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry