Antibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Western blot [3]
- Immunohistochemistry [2]
- Flow cytometry [3]
- Other assay [3]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-41000 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NOXA Monoclonal Antibody (114C307.1)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Suggested positive control: antigen standard for PMAIP1 (transient overexpression lysate), RL-7, Jurkat.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 114C307.1
- Vial size
- 100 μg
- Concentration
- 1.0 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references PUMA and NOXA Expression in Tumor-Associated Benign Prostatic Epithelial Cells Are Predictive of Prostate Cancer Biochemical Recurrence.
Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression.
Chaperone-mediated autophagy promotes lung cancer cell survival through selective stabilization of the pro-survival protein, MCL1.
Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer.
DNA damaging agent-induced apoptosis is regulated by MCL-1 phosphorylation and degradation mediated by the Noxa/MCL-1/CDK2 complex.
Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells.
Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer.
Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells.
BRAF inhibitors suppress apoptosis through off-target inhibition of JNK signaling.
Clairefond S, Péant B, Ouellet V, Barrès V, Tian Z, Trudel D, Karakiewicz PI, Mes-Masson AM, Saad F
Cancers 2020 Oct 29;12(11)
Cancers 2020 Oct 29;12(11)
Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression.
Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, Stein GT, Greninger P, Maves YK, Saunders LR, Dylla SJ, Costa C, Boikos SA, Leverson JD, Souers AJ, Krystal GW, Harada H, Benes CH, Faber AC
Clinical cancer research : an official journal of the American Association for Cancer Research 2018 Jan 15;24(2):360-369
Clinical cancer research : an official journal of the American Association for Cancer Research 2018 Jan 15;24(2):360-369
Chaperone-mediated autophagy promotes lung cancer cell survival through selective stabilization of the pro-survival protein, MCL1.
Suzuki J, Nakajima W, Suzuki H, Asano Y, Tanaka N
Biochemical and biophysical research communications 2017 Jan 22;482(4):1334-1340
Biochemical and biophysical research communications 2017 Jan 22;482(4):1334-1340
Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer.
Nakajima W, Sharma K, Hicks MA, Le N, Brown R, Krystal GW, Harada H
Cancer biology & therapy 2016;17(1):27-35
Cancer biology & therapy 2016;17(1):27-35
DNA damaging agent-induced apoptosis is regulated by MCL-1 phosphorylation and degradation mediated by the Noxa/MCL-1/CDK2 complex.
Nakajima W, Sharma K, Lee JY, Maxim NT, Hicks MA, Vu TT, Luu A, Yeudall WA, Tanaka N, Harada H
Oncotarget 2016 Jun 14;7(24):36353-36365
Oncotarget 2016 Jun 14;7(24):36353-36365
Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells.
Matsumoto M, Nakajima W, Seike M, Gemma A, Tanaka N
Biochemical and biophysical research communications 2016 Apr 29;473(2):490-6
Biochemical and biophysical research communications 2016 Apr 29;473(2):490-6
Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer.
Nakajima W, Hicks MA, Tanaka N, Krystal GW, Harada H
Cell death & disease 2014 Feb 13;5(2):e1052
Cell death & disease 2014 Feb 13;5(2):e1052
Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells.
Pandey MK, Gowda K, Doi K, Sharma AK, Wang HG, Amin S
PloS one 2013;8(11):e78570
PloS one 2013;8(11):e78570
BRAF inhibitors suppress apoptosis through off-target inhibition of JNK signaling.
Vin H, Ojeda SS, Ching G, Leung ML, Chitsazzadeh V, Dwyer DW, Adelmann CH, Restrepo M, Richards KN, Stewart LR, Du L, Ferguson SB, Chakravarti D, Ehrenreiter K, Baccarini M, Ruggieri R, Curry JL, Kim KB, Ciurea AM, Duvic M, Prieto VG, Ullrich SE, Dalby KN, Flores ER, Tsai KY
eLife 2013 Nov 5;2:e00969
eLife 2013 Nov 5;2:e00969
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of NOXA in RL-7 cells (a follicular lymphoma). Sample was incubated in NOXA monoclonal antibody (Product # MA1-41000).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of NOXA in RL-7 cells (a follicular lymphoma). Sample was incubated in NOXA monoclonal antibody (Product # MA1-41000).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot was performed using Anti-NOXA Monoclonal Antibody (114C307.1) (Product # MA1-41000) and 6kDa and 14kDa bands corresponding to NOXA was observed to increase upon IFN-gamma treatment. Whole Cell Extract-WCL (45 µg lysate) of HAEC (Lane 1), HAEC treated with IFN-gamma (50ng/mL for 8Hours) (Lane 2) and HAEC treated with IFN gamma (50ng/mL for 24Hours) (Lane 3) were electrophoresed using Novex™„¢ 16% Tricine Protein Gel (Product # EC6695BOX). Resolved proteins were then transferred onto a PVDF membrane (Product # LC2002) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (2ug/mL dilution) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005). Endogenously, NOXA is expressed at low levels and up-regulates upon apoptotic stimulus. Treatment with IFN-gamma (for 8hrs and 24hrs) increases the expression of ~ 6kDa isoform for progression of downstream cell death signals and forms a ~32 kDa shifted Noxa complex with pHSP27 (the complex is not dependent on IFN gamma treatment and can be seen expressed in control cells also). In addition, Noxa contains 3 identified lysine residues for extensive ubiquitination (mono & poly) which are amplif
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
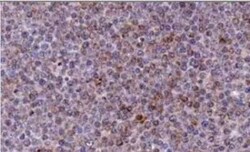
- Experimental details
- Immunohistochemical analysis of NOXA in Bouin-fixed, paraffin-embedded Chronic Lymphocytic Leukemia (CLL) xenograft. Samples were incubated in NOXA monoclonal antibody (Product # MA1-41000) using a dilution of 1:2000 followed by a peroxidase-conjugate and DAB chromogen at a dilution of . Staining for 2 hr at RT. Image using the azide-free form of this antibody.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
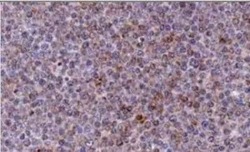
- Experimental details
- Immunohistochemical analysis of NOXA in Bouin-fixed, paraffin-embedded Chronic Lymphocytic Leukemia (CLL) xenograft. Samples were incubated in NOXA monoclonal antibody (Product # MA1-41000) using a dilution of 1:2000 followed by a peroxidase-conjugate and DAB chromogen at a dilution of . Staining for 2 hr at RT. Image using the azide-free form of this antibody.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
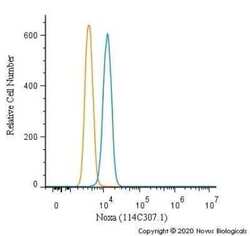
- Experimental details
- Flow cytometry of NOXA in Ntera2 cells. Samples were incubated with NOXA monoclonal antibody (Product # MA1-41000) using a dilution of 1.0 µg/mL for 30 minutes at room temperature followed by Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Dylight 550 (Product # 35503). Antibody (blue) and a matched isotype control (orange). Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
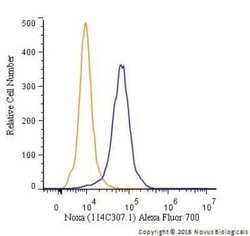
- Experimental details
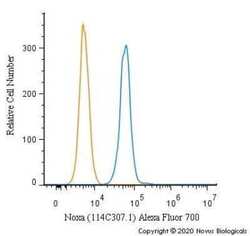
- Flow cytometry of NOXA in HeLa cells. Samples were incubated in NOXA monoclonal antibody (Product # MA1-41000) using a dilution of 5 µg/mL for 30 minutes at room temperature. Antibody (blue) and a matched isotype control (orange). Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin. Both antibodies were conjugated to Alexa Fluor 700.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Flow cytometry of NOXA in MCF7 cells. Samples were incubated in NOXA monoclonal antibody (Product # MA1-41000) using a dilution of 5 µg/mL for 30 minutes at room temperature. Antibody (blue) and a matched isotype control (orange). Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin. Both antibodies were conjugated to Alexa Fluor 700.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
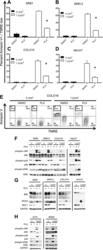
- Figure 1. PLX4720 suppresses UV-induced apoptosis. The cSCC and HaCaT cell lines were either unirradiated or irradiated with 1 kJ/m 2 of UVB in the absence ('o', 1:2000 DMSO) or presence ('+') of 1 muM PLX4720 and isolated for FACS analysis and protein extracts 24 hr later. ( A ) SRB1, ( B ) SRB12, ( C ) COLO16, and ( D ) HaCaT cells show at least 70% suppression of apoptosis in the presence of PLX4720 as measured by FACS for Annexin V+, TMRE-low cells (n = 6 for each cell line, '*' denotes statistical significance at p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
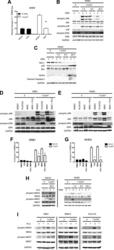
- Figure 2. Vemurafenib and PLX4720 suppress apoptosis and JNK signaling in primary human keratinocytes and cSCC cells independently of MEK/ERK signaling. Normal human epidermal keratinocytes (NHEKs) were irradiated with 1 kJ/m 2 of UVB in the absence ('o', 1:2000 DMSO) or presence ('+') of 1 muM vemurafenib and isolated for FACS analysis and protein extracts 24 hr later. ( A ) Apoptosis was significantly suppressed (70%) in the presence of vemurafenib as measured by FACS for Annexin V+, TMRE-low cells (n = 6, '*' denotes statistical significance at p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
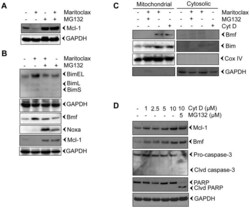
- Figure 3 Maritoclax degrades Mcl-1 by proteasomal activation and releases Bim and Bmf from the cytoskeleton. A-B, Melanoma cells were treated with 5.0 uM of Maritoclax alone or a combination of 5.0 uM Maritoclax and 5 uM MG132 for 12 h and levels of Mcl-1, Bmf, Bim, and Noxa were assessed by immunoblot analysis. C, Melanoma cells were treated with Maritoclax (5.0 uM, 12h) alone, a combination of Maritoclax (5.0 uM) and MG132 (5.0 uM) for 12 h, and cytochalasin D (10 uM, 3h) alone. Subcellular fractions were prepared. Levels of Bmf, Bim, Cox IV, and GAPDH were assessed by immunoblot analysis. D, Melanoma cells were treated with either indicated amount of cytochalasin D or MG132 for 12h. Whole cell extracts were prepared. Levels of Bmf, Mcl-1, Caspase-3, and PARP cleavage were assessed by immunoblot analysis. Loading was confirmed by GAPDH.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot