Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 12-0499-42 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD49d (Integrin alpha 4) Monoclonal Antibody (9F10), PE, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The 9F10 monoclonal antibody reacts with human CD49d, the 150 kDa integrin alpha4 subunit. The complex of CD49d non-covalently associated with integrin beta1 (CD29), also known as VLA-4, is a receptor for fibronectin and VCAM-1 (CD106). This complex is expressed by thymocytes, peripheral lymphocytes, monocytes and eosinophils. CD49d also associates with integrin beta7 and binds to the Mucosal Addressin Cell-Adhesion Molecule-1 (MadCAM-1). Applications Reported: The 9F10 antibody has been reported for use in flow cytometric analysis. Applications Tested: This 9F10 antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (0.125 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 488-561 nm; Emission: 578 nm; Laser: Blue Laser, Green Laser, Yellow-Green Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Conjugate
- Yellow dye
- Isotype
- IgG
- Antibody clone number
- 9F10
- Vial size
- 100 Tests
- Concentration
- 5 μL/Test
- Storage
- 4°C, store in dark, DO NOT FREEZE!
Submitted references Mesenchymal stem cell aggregation mediated by integrin α4/VCAM-1 after intrathecal transplantation in MCAO rats.
Identification of potential chemical compounds enhancing generation of enucleated cells from immortalized human erythroid cell lines.
Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells.
Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin.
Facile Discovery of Cell-Surface Protein Targets of Cancer Cell Aptamers.
Interferon-γ enhances both the anti-bacterial and the pro-inflammatory response of human mast cells to Staphylococcus aureus.
Species-specific differences in the expression and regulation of α4β7 integrin in various nonhuman primates.
Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells.
Lineage depletion of stromal vascular fractions isolated from human adipose tissue: a novel approach towards cell enrichment technology.
Expansion of CMV-mediated NKG2C+ NK cells associates with the development of specific de novo malignancies in liver-transplanted patients.
Tetraspanins CD81 and CD82 facilitate α4β1-mediated adhesion of human erythroblasts to vascular cell adhesion molecule-1.
The immune cell composition in Barrett's metaplastic tissue resembles that in normal duodenal tissue.
Surface antigenic profiling of stem cells from human omentum fat in comparison with subcutaneous fat and bone marrow.
Suppression of tumour-specific CD4⁺ T cells by regulatory T cells is associated with progression of human colorectal cancer.
Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma.
Ran Y, Dong Y, Li Y, Xie J, Zeng S, Liang C, Dai W, Tang W, Wu Y, Yu S
Stem cell research & therapy 2022 Oct 22;13(1):507
Stem cell research & therapy 2022 Oct 22;13(1):507
Identification of potential chemical compounds enhancing generation of enucleated cells from immortalized human erythroid cell lines.
Soboleva S, Kurita R, Ek F, Åkerstrand H, Silvério-Alves R, Olsson R, Nakamura Y, Miharada K
Communications biology 2021 Jun 3;4(1):677
Communications biology 2021 Jun 3;4(1):677
Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells.
Menon V, Thomas R, Elgueta C, Horl M, Osborn T, Hallett PJ, Bartos M, Isacson O, Pruszak J
Stem cells (Dayton, Ohio) 2019 Oct;37(10):1293-1306
Stem cells (Dayton, Ohio) 2019 Oct;37(10):1293-1306
Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin.
Huang NJ, Pishesha N, Mukherjee J, Zhang S, Deshycka R, Sudaryo V, Dong M, Shoemaker CB, Lodish HF
Nature communications 2017 Sep 4;8(1):423
Nature communications 2017 Sep 4;8(1):423
Facile Discovery of Cell-Surface Protein Targets of Cancer Cell Aptamers.
Bing T, Shangguan D, Wang Y
Molecular & cellular proteomics : MCP 2015 Oct;14(10):2692-700
Molecular & cellular proteomics : MCP 2015 Oct;14(10):2692-700
Interferon-γ enhances both the anti-bacterial and the pro-inflammatory response of human mast cells to Staphylococcus aureus.
Swindle EJ, Brown JM, Rådinger M, DeLeo FR, Metcalfe DD
Immunology 2015 Nov;146(3):470-85
Immunology 2015 Nov;146(3):470-85
Species-specific differences in the expression and regulation of α4β7 integrin in various nonhuman primates.
Byrareddy SN, Sidell N, Arthos J, Cicala C, Zhao C, Little DM, Dunbar P, Yang GX, Pierzchalski K, Kane MA, Mayne AE, Song B, Soares MA, Villinger F, Fauci AS, Ansari AA
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 15;194(12):5968-79
Journal of immunology (Baltimore, Md. : 1950) 2015 Jun 15;194(12):5968-79
Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells.
Ruiter B, Patil SU, Shreffler WG
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2015 Jul;45(7):1214-25
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2015 Jul;45(7):1214-25
Lineage depletion of stromal vascular fractions isolated from human adipose tissue: a novel approach towards cell enrichment technology.
Indumathi S, Mishra R, Harikrishnan R, Rajkumar JS, Kantawala N, Dhanasekaran M
Cytotechnology 2014 Mar;66(2):219-28
Cytotechnology 2014 Mar;66(2):219-28
Expansion of CMV-mediated NKG2C+ NK cells associates with the development of specific de novo malignancies in liver-transplanted patients.
Achour A, Baychelier F, Besson C, Arnoux A, Marty M, Hannoun L, Samuel D, Debré P, Vieillard V, K-GREF Study Group
Journal of immunology (Baltimore, Md. : 1950) 2014 Jan 1;192(1):503-11
Journal of immunology (Baltimore, Md. : 1950) 2014 Jan 1;192(1):503-11
Tetraspanins CD81 and CD82 facilitate α4β1-mediated adhesion of human erythroblasts to vascular cell adhesion molecule-1.
Spring FA, Griffiths RE, Mankelow TJ, Agnew C, Parsons SF, Chasis JA, Anstee DJ
PloS one 2013;8(5):e62654
PloS one 2013;8(5):e62654
The immune cell composition in Barrett's metaplastic tissue resembles that in normal duodenal tissue.
Lind A, Siersema PD, Kusters JG, Van der Linden JA, Knol EF, Koenderman L
PloS one 2012;7(4):e33899
PloS one 2012;7(4):e33899
Surface antigenic profiling of stem cells from human omentum fat in comparison with subcutaneous fat and bone marrow.
Dhanasekaran M, Indumathi S, Kanmani A, Poojitha R, Revathy KM, Rajkumar JS, Sudarsanam D
Cytotechnology 2012 Feb 1;64(5):497-509
Cytotechnology 2012 Feb 1;64(5):497-509
Suppression of tumour-specific CD4⁺ T cells by regulatory T cells is associated with progression of human colorectal cancer.
Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G, Gallimore A, Godkin A
Gut 2012 Aug;61(8):1163-71
Gut 2012 Aug;61(8):1163-71
Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma.
Noborio-Hatano K, Kikuchi J, Takatoku M, Shimizu R, Wada T, Ueda M, Nobuyoshi M, Oh I, Sato K, Suzuki T, Ozaki K, Mori M, Nagai T, Muroi K, Kano Y, Furukawa Y, Ozawa K
Oncogene 2009 Jan 15;28(2):231-42
Oncogene 2009 Jan 15;28(2):231-42
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
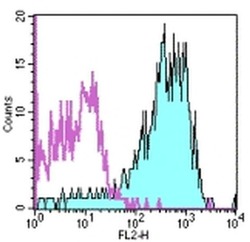
- Experimental details
- Staining of normal human peripheral blood cells with Mouse IgG1 kappa Isotype Control PE (Product # 12-4714-81) (open histogram) or Anti-Human CD49d (Integrin alpha 4) PE (filled histogram). Cells in the lymphocyte gate were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
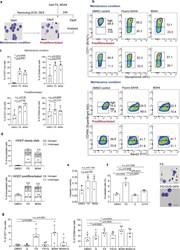
- Experimental details
- Fig. 4 Treatment with HDACi leads to alterations of cell surface phenotype of HiDEP. a Schematic of experimental procedure. HiDEP were treated with FS or M344 for 24 h with or without 5-day pre-differentiation. The cells were then subjected to flow cytometry analyses. b , c Flow cytometry analyses for cell surface expression of representative erythroid cell surface markers. Representative FACS profiles ( b ) and summarized graphs ( c ) are shown. Mean +- SD, n = 4. Significance was determined using one-way ANOVA with Dunnett's multiple comparisons test. d Frequency of enucleated cells after Fluoro-SAHA treatment with or without pre-differentiation treatment. n = 3. Significance was determined using one-way ANOVA with Dunnett's multiple comparisons test. e Measurement of enzymatic activity of caspase-3 in HiDEP treated with DMSO, Fluoro-SAHA (FS), or M344. Mean +- SD, n = 3. Significance was determined using paired t -test. f Effects of caspase inhibition on enucleation of HiDEP upon HDACi treatment. HiDEP were treated with 50 muM of a pan-caspase inhibitor, QVD-OPH, with or without 15 muM of Fluoro-SAHA. Frequency of enucleated cells was analyzed using flow cytometry. FS, Fluoro-SAHA; Q, QVD-OPH. Mean +- SD, n = 3. Significance was determined using one-way ANOVA with Tukey's multiple comparisons test. g Cell surface expression of representative erythroid cell surface markers on HiDEP treated with HDACi with or without QVD-OPH treatment. n = 5. FS, Fluoro-SAHA; Q, QVD-OPH. Mea
- Conjugate
- Yellow dye
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry