Antibody data
- Antibody Data
- Antigen structure
- References [8]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [2]
- Flow cytometry [1]
- Other assay [14]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-1749A - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- ADAMTS4 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-1749A detects ADAMTS4 from porcine, bovine, mouse and human samples. PA1-1749A is specific for ADAMTS4 and shows no cross-reactivity with other ADAMTS family members. PA1-1749A has been successfully used in Western blot procedures. By Western blot, this antibody detects ADAMTS4 at ~68, ~53 and 30 kDa protein representing ADAMTS4 and its proteolytic fragments. Depending on the extent of C-terminal processing, several smaller bands may also be seen using this antibody. PA1-1749A immunogen is a synthetic peptide corresponding to residues C V(394) M A H V D P E E P(403) of mouse ADAMTS4. PA1-1749A immunizing peptide (Cat. # PEP-234) is available for use in neutralization and control procedures. This polyclonal antibody has been referred to as: JSCVMA. PA1-1749A has been used in combination with PA1-1750 for detection of all ADAMTS4 processed forms.
- Reactivity
- Human, Mouse, Bovine, Porcine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references ADAMTS4-specific MR probe to assess aortic aneurysms in vivo using synthetic peptide libraries.
Identification of Adamts4 as a novel adult cardiac injury biomarker with therapeutic implications in patients with cardiac injuries.
Versican is differentially regulated in the adventitial and medial layers of human vein grafts.
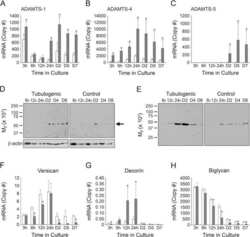
ADAMTS-4 and biglycan are expressed at high levels and co-localize to podosomes during endothelial cell tubulogenesis in vitro.
The anti-atherogenic cytokine interleukin-33 inhibits the expression of a disintegrin and metalloproteinase with thrombospondin motifs-1, -4 and -5 in human macrophages: Requirement of extracellular signal-regulated kinase, c-Jun N-terminal kinase and phosphoinositide 3-kinase signaling pathways.
Tumor necrosis factor-α induces ADAMTS-4 expression in human osteoarthritis chondrocytes.
Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators.
The expression of a disintegrin and metalloproteinase with thrombospondin motifs 4 in human macrophages is inhibited by the anti-atherogenic cytokine transforming growth factor-β and requires Smads, p38 mitogen-activated protein kinase and c-Jun.
Kaufmann JO, Brangsch J, Kader A, Saatz J, Mangarova DB, Zacharias M, Kempf WE, Schwaar T, Ponader M, Adams LC, Möckel J, Botnar RM, Taupitz M, Mägdefessel L, Traub H, Hamm B, Weller MG, Makowski MR
Nature communications 2022 May 23;13(1):2867
Nature communications 2022 May 23;13(1):2867
Identification of Adamts4 as a novel adult cardiac injury biomarker with therapeutic implications in patients with cardiac injuries.
Khanam R, Sengupta A, Mukhopadhyay D, Chakraborty S
Scientific reports 2022 Jun 14;12(1):9898
Scientific reports 2022 Jun 14;12(1):9898
Versican is differentially regulated in the adventitial and medial layers of human vein grafts.
Kenagy RD, Kikuchi S, Evanko SP, Ruiter MS, Piola M, Longchamp A, Pesce M, Soncini M, Deglise S, Fiore GB, Haefliger JA, Schmidt TA, Majesky MW, Sobel M, Wight TN
PloS one 2018;13(9):e0204045
PloS one 2018;13(9):e0204045
ADAMTS-4 and biglycan are expressed at high levels and co-localize to podosomes during endothelial cell tubulogenesis in vitro.
Obika M, Vernon RB, Gooden MD, Braun KR, Chan CK, Wight TN
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2014 Jan;62(1):34-49
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2014 Jan;62(1):34-49
The anti-atherogenic cytokine interleukin-33 inhibits the expression of a disintegrin and metalloproteinase with thrombospondin motifs-1, -4 and -5 in human macrophages: Requirement of extracellular signal-regulated kinase, c-Jun N-terminal kinase and phosphoinositide 3-kinase signaling pathways.
Ashlin TG, Buckley ML, Salter RC, Johnson JL, Kwan AP, Ramji DP
The international journal of biochemistry & cell biology 2014 Jan;46:113-23
The international journal of biochemistry & cell biology 2014 Jan;46:113-23
Tumor necrosis factor-α induces ADAMTS-4 expression in human osteoarthritis chondrocytes.
Xue J, Wang J, Liu Q, Luo A
Molecular medicine reports 2013 Dec;8(6):1755-60
Molecular medicine reports 2013 Dec;8(6):1755-60
Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators.
Krstic D, Rodriguez M, Knuesel I
PloS one 2012;7(10):e47793
PloS one 2012;7(10):e47793
The expression of a disintegrin and metalloproteinase with thrombospondin motifs 4 in human macrophages is inhibited by the anti-atherogenic cytokine transforming growth factor-β and requires Smads, p38 mitogen-activated protein kinase and c-Jun.
Salter RC, Arnaoutakis K, Michael DR, Singh NN, Ashlin TG, Buckley ML, Kwan AP, Ramji DP
The international journal of biochemistry & cell biology 2011 May;43(5):805-11
The international journal of biochemistry & cell biology 2011 May;43(5):805-11
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
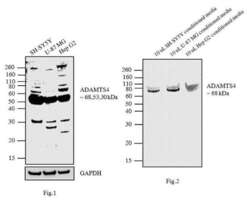
- Experimental details
- Fig1: Western blot analysis was performed using membrane enriched extracts (30 µg lysate) of SH-SY5Y (Lane 1), U-87MG (Lane 2) and Hep G2 (Lane3). Fig2: Western blot analysis was performed using 10 µL conditioned media of SH-SY5Y (Lane 1), U-87 MG (Lane 2) and Hep G2 (Lane3).The blots were probed with ADAMTS4 Rabbit polyclonal Antibody (Product # PA1-1749A, 2 µg/mL) and detected by chemiluminescence using Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, HRP conjugate (Product # A27036, 0.4 µg/mL, 1:2500 dilution). In Fig1. 68, 53 and 30 kDa bands corresponding to ADAMTS4 were observed across the cell lines tested. Along with the desired bands, non-specific bands were observed across cell lines. In Fig.2, ~ 68 kDa band corresponding to ADAMTS4 was observed across the cell lines tested. Known quantity of protein samples were electrophoresed using Novex® NuPAGE® 10 % Bis-Tris gel (Product # NP0302BOX), XCell SureLock™ Electrophoresis System (Product # EI0002) and Novex® Sharp Pre-Stained Protein Standard (Product # LC5800). Resolved proteins were then transferred onto a nitrocellulose membrane with iBlot® 2 Dry Blotting System (Product # IB21001). The membrane was probed with the relevant primary and secondary Antibody following blocking with 5% skimmed milk. Chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
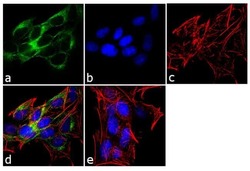
- Experimental details
- Immunofluorescence analysis of ADAMTS4 was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with ADAMTS4 Rabbit Polyclonal Antibody (Product # PA1-1749A) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
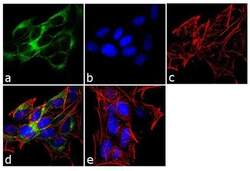
- Experimental details
- Immunofluorescence analysis of ADAMTS4 was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with ADAMTS4 Rabbit Polyclonal Antibody (Product # PA1-1749A) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
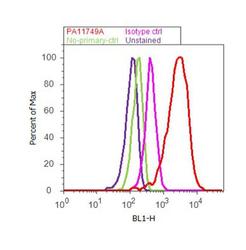
- Experimental details
- Flow cytometry analysis of ADAMTS4 was performed using SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with ADAMTS4 Rabbit Polyclonal Antibody (PA1-1749A, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control..
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
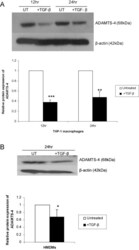
- Fig. 2 TGF-beta inhibits ADAMTS-4 protein expression in macrophages. THP-1 macrophages (A) or HMDMs (B) were either left untreated (UT) or incubated with 30 ng/ml TGF-beta for the time points indicated. An equal amount of the protein extract was subjected to Western blot analysis using antisera against ADAMTS-4 and the control beta-actin, as shown. The relative ADAMTS-4 expression normalised to beta-actin, as determined by densitometric analysis, from three independent experiments is shown (mean +- SD) with the values from untreated cells arbitrarily assigned as 1 (* P < 0.05; ** P < 0.01; *** P < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
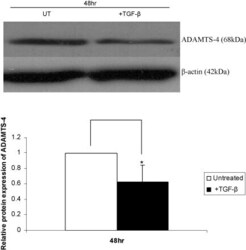
- Fig. 3 TGF-beta inhibits ADAMTS-4 protein expression in Hep3B cells. The cells were either left untreated (UT) or incubated with TGF-beta for 48 h. Equal amounts of protein were subjected to Western blot analysis using antisera against ADAMTS-4 and the control beta-actin, as indicated. The relative ADAMTS-4 expression normalised to beta-actin, as determined by densitometric analysis, from three independent experiments is shown (mean +- SD) with the value from untreated cells arbitrarily assigned as 1 (* P < 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
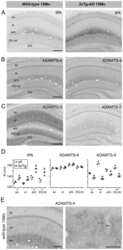
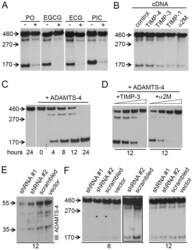
- Figure 2 ADAMTS-4 cleaves Reelin at both the N- and C-terminus. ( A-D, F ) Anti-Reelin (G10, N-terminal antibody) immunoblots (IB). ( A,B ) Samples from Reelin-expressing HEK293 cells were collected after 12 hours incubation. ( A ) Metal chelator (PO, 50 uM), catechins (EGCG, 150 uM; ECG, 150 uM), and picetanol (PIC, 60 uM) inhibit Reelin cleavage. ( B ) Expression of TIMP-3, -1, and alpha2M, but not TIMP-4, inhibit Reelin cleavage. ( C ) Recombinant ADAMTS-4 (10 ng/ul) processes Reelin at both cleavage sites. Short vertical line at the bottom of the blot denotes that the last lanes, from the same blot, were joined for visual presentation. ( D ) Recombinant TIMP-3 (1.5, 3, 6, 12 ng/ul) and alpha2M (44, 72, 88,100 ng/ul) inhibit the proteolytic action of ADAMTS-4 on full-length Reelin. ( E ) Anti-ADAMTS-4 immunoblots (IB) showing reduction of ADAMTS-4 protein levels in HeLa cells transfected with two different ADAMTS-4 shRNA constructs, but not with scrambled shRNA nor with empty vector. ( F ) ADAMTS-4 shRNA transiently inhibited Reelin cleavage in Reelin-expressing HeLa cells. The frame emcompasses overexposed lanes from the blot on the right (asterisks) for better visualization of the Reelin fragments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
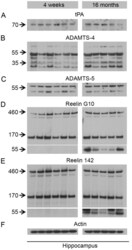
- Figure 5 Biochemical analysis of Reelin preoteolytic processing and protein levels of the identified Reelin proteases in young and old wild-type mice. Immunoblots using ( A ) anti-tPA (H-90), ( B ) anti-ADAMTS-4 (PA1-1749A), ( C ) anti-ADAMTS-5 (ab41037), ( D ) anti-Reelin (G10), ( E ) anti-Reelin (142), ( F ) anti-Actin (MAB1522) antibody. Lanes represent different animals. Hippocampus lysates from young (4 weeks) and old (16 months) animals were processed on the same gel and membrane. No difference in Reelin cleavage or levels of Reelin proteases is observed between young and old animals. However, a prominent Reelin-positive band of ~60 kDa was selectively observed in the aged animals.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
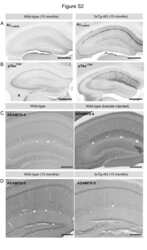
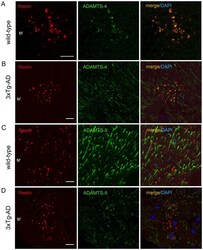
- Figure 7 Co-localization of ADMTS-4 and Reelin in the striatum radiatum of aged animals. ( A-D ) Double-immunofluorescence staining using anti-Reelin (red) and anti- ADAMTS-4 and anti-ADAMTS-5 (green) antibodies on brain sections of 15 month-old non-transgenic and 3xTg-AD mice. Co-localization analysis revealed a selective overlap of ADAMTS-4 IR ( A,B ), but not ADAMTS-5 IR ( C,D ), with Reelin in extracellular deposits in the striatum radiatum (sr). DAPI (blue) counterstaining was used for labeling the cell nuclei. Scale bars: 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
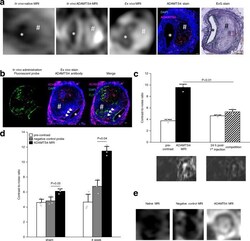
- Confirmation of the specific binding of the ADAMTS4-specific probe. a From left to right: In vivo MRI before and after administration of the ADAMTS4-specific probe in one male mouse 4 weeks following aneurysms induction was highly comparable to corresponding ex vivo MRI scan of the abdominal aorta and ex vivo immunofluorescence staining (ADAMTS4 stain) and Elastica van Gieson (EvG) staining. b Intravenous administration of the fluorescent variant of the ADAMTS4-specific probe resulted in a strong fluorescence of the aneurysmal wall that was clearly co-localized with areas of ADAMTS4 expression using immunofluorescence staining (white arrows). c The competition experiment ( n = 3 mice) showed a significant decrease in the contrast-to-noise-ratio compared to the administration of the gadolinium-labeled ADAMTS4-specific probe alone, confirming the specific binding of the probe ( P = 0.0025). Data are presented as mean values +- SEM. d , e Using the negative control probe Cys*-Phe-His-Pro-Tyr-Cys*-linker (DOTA-Gd) with a similar size to the ADAMTS4-specific probe, no significant increase in contrast-to-noise ratio (CNR) was measured in vivo in the aortic wall of ApoE -/- mice ( n = 6). The application of the ADAMTS4-specific probe resulted in a strong and significant increase in CNR in the vessel wall as well in the thrombus. * P = 0.08; ** P = 0.04; Data are presented as mean values +- SEM. FLP Fluorescein-labeled probe, Scale bars indicate 100 um. * indicate the aortic lumen; #
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
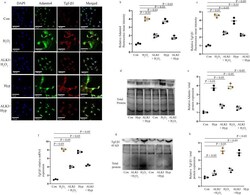
- Adamts4 and Tgf-beta1 expression is inhibited following pre-treatment with ALKI. Staining with anti-Adamts4 (shown in green) showed a 4- and 3.7-fold increase following H 2 O 2 and hypoxia treatments in the fluorescence intensities of Adamts4 but was found to reduce to 2 and 1.6 folds in the treatment groups--ALKI + H 2 O 2 and ALKI + Hypoxia respectively ( a and b ). IF with anti-Tgf-beta1 antibody (shown in red) showed elevated expression of 4.5 and fourfold following H 2 O 2 and hypoxia treatments and this reduced to 2.5 and 2.3 folds for ALKI + H 2 O 2 and ALKI + Hypoxia groups ( a and c ). DAPI (in blue) was used as nuclear stain. Adamts4 WB shows of increased expression of 3.7 and 4 folds for the Hyp, H 2 O 2 treated groups which reduce to 1.7, and 1.5 in ALKI + Hyp and ALKI + H 2 O 2 treated groups ( d and e ). Tgf-beta1 inhibition following ALKI pre-treatment was also assessed by qPCR ( f ) which showed a reduction from 8 and 7.4 folds for H 2 O 2 and hypoxia treatment groups to 4 and 4.4 folds for ALKI + H 2 O 2 and ALKI + Hyp treatment groups. Tgf-beta1 protein expression measured by WB ( g and h ) showed a 4- and 3.8-fold increased change for H 2 O 2 and Hypoxia subjected H9c2 cells to 1.5 and 1.8 folds for ALKI + H 2 O 2 and ALKI + Hyp treatment respectively. beta-actin was used to normalize gene expression for qPCR assay and total protein was used as loading control for WB. n = 3, data analyzed and expressed as mean +- SD. Differences were considered statisticall
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
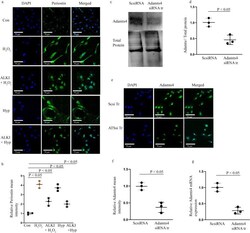
- Periostin expression is reduced after ALKI pre-treatment and successful knockdown of Adamt4 mRNA in cultured H9c2 cells. IF with anti-Periostin antibody (shown in green) shows inhibition of Periostin expression under ALKI + H 2 O 2 , and ALKI + Hyp conditions as compared to only H 2 O 2 and Hyp treated conditions. A reduced expression of 2.3 and 2 folds for ALKI + H 2 O 2 , and ALKI + Hyp was observed which was a decrease from the 4 and 3.7 fold change found for only H 2 O 2 and Hyp treatments ( a and b ) in comparison to control. Successful knockdown of Adamts4 via Adamts4 siRNA transfection is shown by Adamts4 WB (shown in c and d ), a 0.46 fold expression in the knockdown group against the control group was found. Further, Adamts4 IF (shown in green) also validated the successful knockdown of Adamts4. A decrease in the mean fluorescence intensity from 1 as observed for Scsi treatment set to 0.33 for ATSsi tr group was found. ( e and f ). DAPI (shown in blue) was used as nuclear stain. Finally, from qPCR Adamts4 mRNA levels were downregulated from onefold observed for Scsi tr set to 0.25-fold for ATSsi tr group ( g ). n = 3, data analyzed and expressed as mean +- SD. Differences were considered statistically significant for p < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
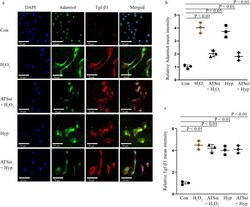
- Tgf-beta1 expression remains unaffected by Adamts4 knockdown. IF with anti-Adamts4 (shown in green) showed downregulation of Adamts4 following Adamts4 loss of function along with injury treatments. Adamts4 levels were found to decrease to 2 and 1.8 folds in the ATSsi + H 2 O 2 and ATSsi + Hyp groups in comparison to the 4 and 3.7 folds increase observed for only H 2 O 2 and Hyp treatments ( a and b ) but Tgf-beta1 remained largely unaffected by this loss of function of Adamts4 and as shown by staining with anti-Tgf-beta1 (shown in red) where the levels of Tgf-beta1 were 4.5 and 4.0 for H 2 O 2 and Hyp groups and 4.2 and 4.1 for ATSsi + H 2 O 2 and ATSsi + Hyp groups (a and c). DAPI (shown in blue) was used as nuclear stain. Differences between groups H 2 O 2 and ATSsi + H 2 O 2 , and Hyp and ATSsi + Hyp were not found to be significant. n = 3, data analyzed and expressed as mean +- SD. Differences were considered statistically significant for p < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
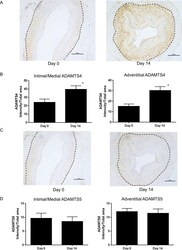
- Experimental details
- Fig 2 ADAMTS4 and 5 immunostaining in vein rings. (A) ADAMTS4 immunostaining in pre- and 14 day cultured floating vein rings was quantified (B) for the intima/media and adventitia (* P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
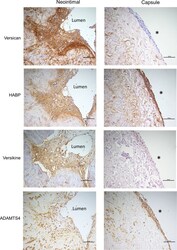
- Experimental details
- Fig 6 Immunostaining of cells in the neointima and adventitial capsule. Histochemical and immunostaining of neointimal cells and capsule cells (left and right panel, respectively) for (A) versican, (B) hyaluronan, (C) versikine, and (D) ADAMTS4. * indicates the adventitial surface. Scale bars indicate 100 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot