Antibody data
- Antibody Data
- Antigen structure
- References [13]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunoprecipitation [1]
- Other assay [18]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 36-4100 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- ZO-3 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling.
Angulin-1 seals tricellular contacts independently of tricellulin and claudins.
Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes.
Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity.
Cell-specific diversity in the expression and organization of cytoplasmic plaque proteins of apical junctions.
Claudin-2 knockout by TALEN-mediated gene targeting in MDCK cells: claudin-2 independently determines the leaky property of tight junctions in MDCK cells.
ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape.
Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion.
ZO proteins redundantly regulate the transcription factor DbpA/ZONAB.
Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia.
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
An optimized in vitro model of the respiratory tract wall to study particle cell interactions.
A three-dimensional cellular model of the human respiratory tract to study the interaction with particles.
Yang Y, Fan X, Ji Y, Li J, Dai Z, Wu Z
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):1-9
Animal nutrition (Zhongguo xu mu shou yi xue hui) 2022 Mar;8(1):1-9
Angulin-1 seals tricellular contacts independently of tricellulin and claudins.
Sugawara T, Furuse K, Otani T, Wakayama T, Furuse M
The Journal of cell biology 2021 Sep 6;220(9)
The Journal of cell biology 2021 Sep 6;220(9)
Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes.
Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z, Wu Z
Nutrients 2021 Jan 26;13(2)
Nutrients 2021 Jan 26;13(2)
Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity.
Otani T, Nguyen TP, Tokuda S, Sugihara K, Sugawara T, Furuse K, Miura T, Ebnet K, Furuse M
The Journal of cell biology 2019 Oct 7;218(10):3372-3396
The Journal of cell biology 2019 Oct 7;218(10):3372-3396
Cell-specific diversity in the expression and organization of cytoplasmic plaque proteins of apical junctions.
Vasileva E, Sluysmans S, Bochaton-Piallat ML, Citi S
Annals of the New York Academy of Sciences 2017 Oct;1405(1):160-176
Annals of the New York Academy of Sciences 2017 Oct;1405(1):160-176
Claudin-2 knockout by TALEN-mediated gene targeting in MDCK cells: claudin-2 independently determines the leaky property of tight junctions in MDCK cells.
Tokuda S, Furuse M
PloS one 2015;10(3):e0119869
PloS one 2015;10(3):e0119869
ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape.
Tokuda S, Higashi T, Furuse M
PloS one 2014;9(8):e104994
PloS one 2014;9(8):e104994
Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion.
Qiao X, Roth I, Féraille E, Hasler U
Cell cycle (Georgetown, Tex.) 2014;13(19):3059-75
Cell cycle (Georgetown, Tex.) 2014;13(19):3059-75
ZO proteins redundantly regulate the transcription factor DbpA/ZONAB.
Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S
The Journal of biological chemistry 2014 Aug 8;289(32):22500-11
The Journal of biological chemistry 2014 Aug 8;289(32):22500-11
Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia.
Fanning AS, Van Itallie CM, Anderson JM
Molecular biology of the cell 2012 Feb;23(4):577-90
Molecular biology of the cell 2012 Feb;23(4):577-90
Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells.
Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, Fukamachi H, Ito Y
Gastroenterology 2010 Jan;138(1):255-65.e1-3
Gastroenterology 2010 Jan;138(1):255-65.e1-3
An optimized in vitro model of the respiratory tract wall to study particle cell interactions.
Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P
Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine 2006 Fall;19(3):392-405
Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine 2006 Fall;19(3):392-405
A three-dimensional cellular model of the human respiratory tract to study the interaction with particles.
Rothen-Rutishauser BM, Kiama SG, Gehr P
American journal of respiratory cell and molecular biology 2005 Apr;32(4):281-9
American journal of respiratory cell and molecular biology 2005 Apr;32(4):281-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
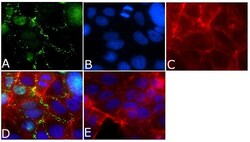
- Experimental details
- Immunofluorescent analysis of ZO-3/TJP3 Antibody was done on 90% confluent log phase CACO2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with ZO-3/TJP3 Antibody (Product # 36-4100) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cell junctional localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of ZO-3/TJP3 Antibody was done on 90% confluent log phase CACO2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with ZO-3/TJP3 Antibody (Product # 36-4100) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cell junctional localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
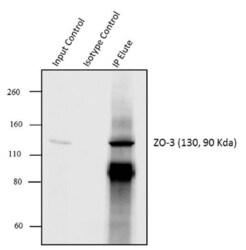
- Experimental details
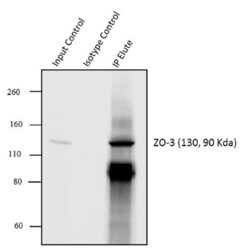
- Immunoprecipitation of ZO-3 was performed with 5 µg of the ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100) on cell extract from MCF7 using the Dynabeads® Protein A Immunoprecipitation Kit (10006D). Normal Rabbit IgG was used as a negative IP control. Subsequently, western blot analysis was performed using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0322BOX), XCell SureLock™ Electrophoresis System (Product # EI0002), Novex® Sharp Pre-Stained Protein Standard (LC5800). Proteins were transferred using Pierce™ G2 Fast Blotter (22834SPCL) to a nitrocellulose membrane and blocked with 5% skim milk for 1 hour at room temperature on a rocking platform. ZO-3 was detected at ~130 kDa using ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100) at 1-2 µg/mL in 5 % skim milk at 4°C overnight on a rocking platform. Goat Anti-Rabbit IgG - HRP Secondary Antibody (G21234) at 1:5000 dilution was used and chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106). Lane 1 represents 10% of the total cell extract (input), Lane 2 is the IP performed with Rabbit IgG and Lane 3 represents IP performed with ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
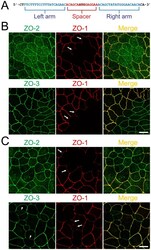
- Figure 1 Construction of TALENs and ZO-1 gene knockout in MDCK I and II cells. (A) TALEN binding sites in the ZO-1 gene. The left and right arms of TALEN targeting sites are indicated in blue and the spacer region is indicated in red. The initiation codon within the spacer region is highlighted. (B) Immunofluorescence microscopic analysis of ZO-1, ZO-2 and ZO-3 in MDCK II cells transfected with TALEN constructs for ZO-1 gene knockout. After transfection, cells were subcultured on filter inserts for 4 d before analysis. At the boundary of control and ZO-1 knockout cells, characteristic convex curves of cell-cell junctions are observed ( arrows ). (C) Immunofluorescence microscopic analysis of ZO-1, ZO-2 and ZO-3 in MDCK I cells transfected with TALEN constructs for ZO-1 gene knockout. Similar morphological changes of cell-cell junctions at the boundary of control and ZO-1 knockout cells were observed in MDCK I cells. Staining of ZO-3 was reduced in ZO-1 knockout cells ( arrowheads ). Scale bars, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 3 Knockout of ZO-2 or ZO-3 in MDCK II cells. (A) TALEN binding sites in ZO-2 and ZO-3 genes. The left and right arms of TALEN targeting sites are indicated in blue and the spacer region is indicated in red. The initiation codon within the spacer region is highlighted. (B) Immunofluorescence microscopic analysis of ZO proteins in MDCK II cells transfected with TALEN constructs for ZO-2 or ZO-3 gene knockout. The shape of cell-cell junctions was unchanged by ZO-2 knockout (upper panels) and ZO-3 knockout (lower panels). Scale bar, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
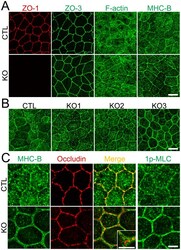
- Figure 5 Effects of ZO-1 knockout on the shape of cell-cell junctions and cytoskeleton. (A) Immunofluorescence microscopic analysis of ZO-1, ZO-3, F-actin and myosin heavy chain II-B (MHC-B) in control MDCK II cells and ZO-1 knockout clone. ZO-1 knockout induced striking changes in MHC-B organization. Scale bar, 10 um. (B) Immunofluorescence microscopic analysis of MHC-B in control MDCK II cells and ZO-1 knockout clones. Similar punctate staining of MHC-B at cell-cell contacts was observed in all three ZO-1 knockout clones. Scale bar, 10 um. (C) Immunofluorescence microscopic analysis of MHC-B, occludin and 1-phosphomyosin light chain (1p-MLC) at high magnification. Coimmunolocalization of MHC-B and occludin revealed punctate staining of MHC-B was arranged in two rows along the cell-cell junctions across the staining of occludin. 1p-MLC was concentrated at cell-cell contacts in ZO-1 knockout cells. Scale bar, 5 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
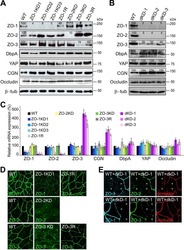
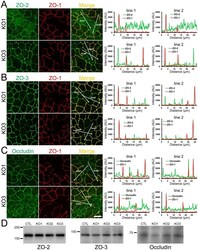
- Figure 6 Effects of ZO-1 knockout on the localization of ZO-2, ZO-3 and occludin in the stable ZO-1 knockout clones. (A) Immunofluorescence microscopic analysis and line scanning of ZO-1 and ZO-2 in ZO-1 knockout clones 1 (KO 1) and 3 (KO 3) co-cultured with control cells. ZO-2 fluorescent signal at TJs was reduced in KO 1 but was slightly increased in KO 3. (B) Immunofluorescence microscopic analysis and line scanning of ZO-1 and ZO-3. ZO-3 signal at TJs was reduced but was increased in the cytoplasm in both of ZO-1 knockout clones compared to co-cultured control cells. (C) Immunofluorescence microscopic analysis and line scanning of ZO-1 and occludin. Occludin signal at TJs was reduced in both of ZO-1 knockout clones compared to co-cultured control cells. Scale bar, 10 um. (D) Immunoblots of ZO-2, ZO-3 and occludin in control MDCK II cells and ZO-1 knockout clones. Similar expression levels of ZO-2, ZO-3 and occludin were observed in control cells and knockout clones.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
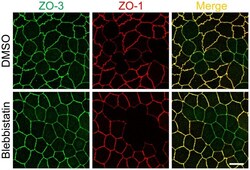
- Figure 7 Effects of blebbistatin on the shape of cell-cell junctions in ZO-1 knockout cells. Effects of blebbistatin on the shape of cell-cell junctions in the co-culture of control and ZO-1 knockout cells. Cells were treated with 50 uM blebbistatin for 2 h and analyzed by immunofluorescence microscopy. Blebbistatin treatment attenuated the curves of cell-cell junctions at the boundary of the control and ZO-1 knockout cells, and the shape of cell-cell junctions in the control and ZO-1 knockout cells were almost indistinguishable in blebbistatin treated cells. Scale bar, 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
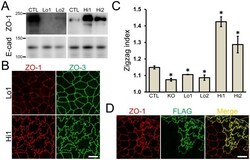
- Figure 8 Effects of the amount of ZO-1 expression on the shape of cell-cell junctions. (A) Immunoblots of ZO-1 and E-cadherin in control MDCK II cells and ZO-1 knockout clones expressing exogenous ZO-1. FLAG-tagged ZO-1 was transfected into ZO-1 knockout cells, and clones expressing a nominal amount of ZO-1 (Lo 1 and 2) and an excessive amount of ZO-1 (Hi 1 and 2) were established. The bands of ZO-1 from Lo 1 and 2 clones were detected at very low levels in the enhanced image of immunoblots incubated with anti-ZO-1 antibody. (B) Immunofluorescence microscopic analysis of ZO-1 and ZO-3 in clones Lo 1 and Hi 1. Intensive zigzag shape of cell-cell junctions was observed in Hi 1 clone. Scale bar, 10 um. (C) Effect of ZO-1 expression levels on the shape of cell-cell junctions. The degree of zigzag of cell-cell junctions was quantified in each clone and presented as zigzag index. The zigzag index was significantly lower in ZO-1 knockout clones and Lo 1 and 2 clones, and was significantly higher in Hi 1 and 2 clones compared with control cells. Data are shown as means +- S.E. N = 3-4 for each clone. *, p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunoprecipitation of ZO-3 was performed with 5 µg of the ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100) on cell extract from MCF7 using the Dynabeads® Protein A Immunoprecipitation Kit (10006D). Normal Rabbit IgG was used as a negative IP control. Subsequently, western blot analysis was performed using Novex® NuPAGE® 4-12 % Bis-Tris gel (Product # NP0322BOX), XCell SureLock™ Electrophoresis System (Product # EI0002), Novex® Sharp Pre-Stained Protein Standard (LC5800). Proteins were transferred using Pierce™ G2 Fast Blotter (22834SPCL) to a nitrocellulose membrane and blocked with 5% skim milk for 1 hour at room temperature on a rocking platform. ZO-3 was detected at ~130 kDa using ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100) at 1-2 µg/mL in 5 % skim milk at 4°C overnight on a rocking platform. Goat Anti-Rabbit IgG - HRP Secondary Antibody (G21234) at 1:5000 dilution was used and chemiluminescent detection was performed using Pierce™ ECL Western Blotting Substrate (Product # 32106). Lane 1 represents 10% of the total cell extract (input), Lane 2 is the IP performed with Rabbit IgG and Lane 3 represents IP performed with ZO-3 Rabbit Polyclonal Antibody (Product # 36-4100).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
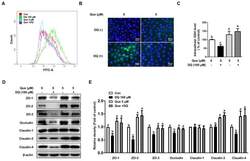
- Figure 3 Quercetin attenuated diquat-induced ROS accumulation and reduced protein abundance of tight junction proteins in IPEC-1 cells. IPEC-1 cells were treated as described in Figure 2 . ( A ) DCFH-DA-positive populations were detected by flow cytometry analysis. ( B ) Intracellular ROS levels were determined by fluorescence microscope (magnification x200). ( C ) Intracellular GSH levels were measured. ( D , E ) Protein abundance for ZO-1, ZO-2, ZO-3, occludin, claudin-1, claudin-3, and claudin-4 were determined and analyzed by the Western blot assay. beta-actin was used as the loading control. Representative experimental results were repeated in three independent experiments and values are means +- SEMs, n = 3. Means without a common letter are considered as a significant difference, p < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
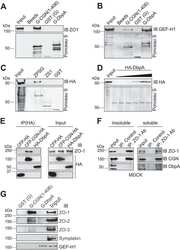
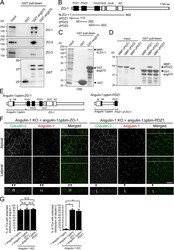
- Figure 7. Direct binding of the angulin-1 pbm to ZO-1 and its role in tTJ formation. (A) Lysates of angulin-1-KO cells were incubated with GST and GST fusion proteins of aa 409-575 and aa 409-570 of angulin-1 (GST-ang575 and GST-ang570, respectively) and subjected to GST pull-down assays. The lysates (input) and precipitates with GST, GST-ang575, and GST-ang570 were analyzed by Western blotting with anti-ZO-1 mAb, anti-ZO-2 pAb, and anti-ZO-3 pAb. The precipitates were also immunoblotted with anti-GST pAb. (B) Schematic diagram of the domain structure of mouse ZO-1 protein with 1,745 amino acids. ZO-1 contains three PDZ domains, an SH3 domain, a guanylate kinase domain (GUK), and an acidic domain (AD) in its N-terminal half. Four distinct portions of ZO-1 indicated as solid lines with amino acid numbers were produced as recombinant fusion proteins with MBP. (C) GST pull-down assays of MBP-N-ZO-1 fusion protein with GST-ang575. Input: crude lysate of Escherichia coli expressing MBP-N-ZO-1. (D) GST pull-down assays of MBP fusion proteins of PDZ1, PDZ2, and PDZ3 of ZO-1 (MBP-zPDZ1, MBP-zPDZ2, and MBP-zPDZ3, respectively) with GST-ang575. Input: each MBP fusion protein. In C and D, the samples were subjected to SDS-PAGE followed by Coomassie Brilliant Blue (CBB) staining. (E) Schematic diagram of a chimeric protein of angulin-1Deltapbm with full-length ZO-1 (angulin-1Deltapbm-ZO-1) and a chimeric protein of angulin-1Deltapbm with PDZ1 domain of ZO-1 (angulin-1Deltapbm-PDZ1). (F)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
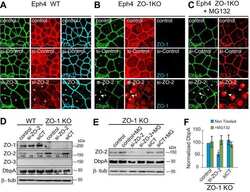
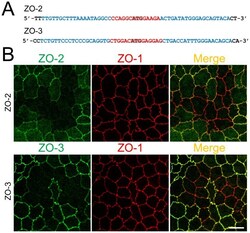
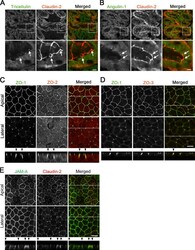
- Figure S1. Localization of TJ and tTJ proteins at TCs. (A and B) Double-immunofluorescence staining of frozen mouse kidney sections containing proximal tubules with anti-tricellulin mAb and anti-claudin-2 pAb (A) and anti-angulin-1 mAb and anti-claudin-2 pAb (B). Tricellulin and angulin-1 showed dotlike staining at TCs with claudin-2 staining (arrows). (C-E) Double-immunofluorescence staining of MDCK II cells with anti-ZO-1 mAb and anti-ZO-2 pAb (C), anti-ZO-1 mAb and anti-ZO-3 pAb (D), and anti-JAM-A pAb and anti-claudin-2 mAb (E). Confocal sections in the apical region, including TJ markers and the lateral region, together with the corresponding Z-stack images along the white dotted lines are shown. Arrowheads indicate TCs. Bars: 10 um (top of A and B and C-E), 5 um (bottom of A and B).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
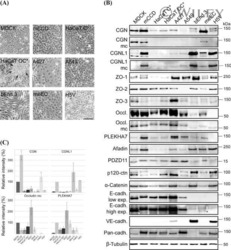
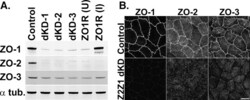
- FIGURE 1: ZO-1 and -2 are effectively depleted in stable MDCK II Tet-Off cell lines. (A) Western blot of ZO-1, -2, and -3 polypeptides in control, dKD (lines 1-3), and in dKD cells expressing a Tet-inducible full-length ZO-1 rescue transgene (ZO1R). ZO-1 expression in dKD lines is ~2-3% of that observed in control cells, while ZO-2 expression is almost undetectable. Note that ZO-3, while not directly targeted in dKD cells, is also reduced by up to 50%. In ZO1R cell lines, induction of ZO1R (I) restores ZO-1 and ZO-3 expression to levels equivalent to those in control cells, while ZO-2 expression remains suppressed. U, uninduced; I, induced. (B) Immunocytochemistry of ZO-1, -2, and -3 in the control and dKD-3 cell line. There is a dramatic reduction in the staining of all three peptides at the AJC, and the outline of the AJC is much more trapezoidal in dKD cells relative to control cells. All images are 1-mum-thick, maximum-density projections of the AJC. Scale bar: 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
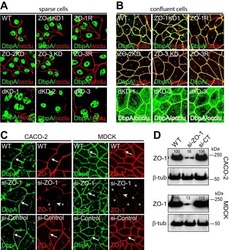
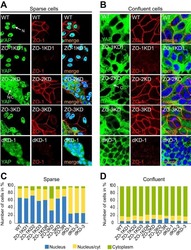
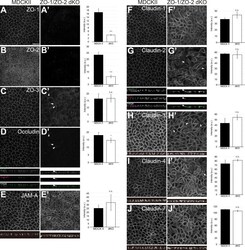
- Figure 1. ZO-1/ZO-2 are required for apical junction localization of TJ proteins. (A-J) Immunofluorescence analyses of parental MDCK II cells (A-J) and ZO-1/ZO-2 dKO cells (A'-J'). (A and B) ZO-1 (A) and ZO-2 (B) expression was abolished in ZO-1/ZO-2 dKO cells. (C-J) TJ markers including ZO-3 (C), occludin (D), JAM-A (E), claudin-1 (F), claudin-2 (G), claudin-3 (H), claudin-4 (I), and claudin-7 (J) were not concentrated to the apical junctions in ZO-1/ZO-2 dKO cells, and JAM-A (E) and claudins (F-J) were diffusely localized along the lateral and apical plasma membrane. Occasional apical junction accumulation of occludin and claudins, but not JAM-A was observed (arrowheads), colocalizing with ZO-3 (z-sections in D and G). Graphs are quantitation of the fluorescence intensity and represent mean +- SD ( n = 2-9). *, P < 0.05; ***, P < 0.0005, compared by t test. Scale bar: 20 um. n.s., not significant.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry