Antibody data
- Antibody Data
- Antigen structure
- References [52]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [3]
- Flow cytometry [3]
- Other assay [24]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-027A - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cyclophilin B Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-027A detects cyclophilin B (CyPB) in mouse, rat, canine, and human tissues. PA1-027A has been successfully used in Western blot procedures, IF/ICC and IHC (P). By Western blot, this antibody detects a ~19 kDa protein representing CyPB from rat liver extract. The PA1-027A immunogen is a synthetic peptide corresponding to residues C(194) G K I E V E K P F A I A K E(208) of human CyPB. This sequence is almost completely conserved between human, mouse, rat, bovine, and chicken. PA1-027A immunizing peptide (Cat. # PEP-058) is available for use in neutralization and control experiments.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Mitofusin 1 and 2 regulation of mitochondrial DNA content is a critical determinant of glucose homeostasis.
Phosphorylation of CREB at Serine 142 and 143 Is Essential for Visual Cortex Plasticity.
Proteomics reveal cap-dependent translation inhibitors remodel the translation machinery and translatome.
Amelioration of Murine Macrophage Activation Syndrome by Monomethyl Fumarate in Both a Heme Oxygenase 1-Dependent and Heme Oxygenase 1-Independent Manner.
Structure of the full-length human Pannexin1 channel and insights into its role in pyroptosis.
TAPBPR promotes antigen loading on MHC-I molecules using a peptide trap.
Distinct states of proinsulin misfolding in MIDY.
Tumor suppressive function of Matrin 3 in the basal-like breast cancer.
Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication.
MAFG-driven astrocytes promote CNS inflammation.
Loss of tyrosine kinase receptor Ephb2 impairs proliferation and stem cell activity of spermatogonia in culture†.
Cyclophilin B control of lysine post-translational modifications of skin type I collagen.
TRIM5α Restricts Flavivirus Replication by Targeting the Viral Protease for Proteasomal Degradation.
Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes β-catenin.
Disruption of the β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner.
Target-Based Screening against eIF4A1 Reveals the Marine Natural Product Elatol as a Novel Inhibitor of Translation Initiation with In Vivo Antitumor Activity.
Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence.
Long noncoding RNA MALAT1 suppresses breast cancer metastasis.
The Role of CREB, SRF, and MEF2 in Activity-Dependent Neuronal Plasticity in the Visual Cortex.
Expression of Peroxisome Proliferator-Activated Receptor alpha (PPARα) in somatotropinomas: Relationship with Aryl hydrocarbon receptor Interacting Protein (AIP) and in vitro effects of fenofibrate in GH3 cells.
Furin Cleavage of L2 during Papillomavirus Infection: Minimal Dependence on Cyclophilins.
miR-100 induces epithelial-mesenchymal transition but suppresses tumorigenesis, migration and invasion.
Thyroid hormone and estradiol have overlapping effects on kidney glutathione S-transferase-α gene expression.
LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker.
Thyroid hormone contributes to the hypolipidemic effect of polyunsaturated fatty acids from fish oil: in vivo evidence for cross talking mechanisms.
Blocking leptin action one week after weaning reverts most of the programming caused by neonatal hyperleptinemia in the adult rat.
Protein kinase C activity regulates D-serine availability in the brain.
Activation of D1/D5 dopamine receptors protects neurons from synapse dysfunction induced by amyloid-beta oligomers.
Liver glutathione S-transferase expression is decreased by 3,5,3-triiodothyronine in hypothyroid but not in euthyroid mice.
Small interfering RNA knocks down the molecular target of alendronate, farnesyl pyrophosphate synthase, in osteoclast and osteoblast cultures.
Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation.
Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes.
Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I.
Cyclophilin B interacts with sodium-potassium ATPase and is required for pump activity in proximal tubule cells of the kidney.
Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation.
siRNA knock-down of RANK signaling to control osteoclast-mediated bone resorption.
Calcineurin signaling regulates human islet {beta}-cell survival.
Amyloid-β triggers the release of neuronal hexokinase 1 from mitochondria.
Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation.
Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF.
Soluble oligomers from a non-disease related protein mimic Abeta-induced tau hyperphosphorylation and neurodegeneration.
Characterization of hepatitis C virus subgenomic replicon resistance to cyclosporine in vitro.
Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion.
The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter.
Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins.
Role of cyclophilin B in activation of interferon regulatory factor-3.
Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins.
The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system. Characterization of the system in normal and warfarin-resistant rats.
High binding capacity of cyclophilin B to chondrocyte heparan sulfate proteoglycans and its release from the cell surface by matrix metalloproteinases: possible role as a proinflammatory mediator in arthritis.
Transport activity and surface expression of the Na+-Ca2+ exchanger NCX1 are inhibited by the immunosuppressive agent cyclosporin A and by the nonimmunosuppressive agent PSC833.
Selective intracellular retention of extracellular matrix proteins and chaperones associated with pseudoachondroplasia.
Selective intracellular retention of extracellular matrix proteins and chaperones associated with pseudoachondroplasia.
Sidarala V, Zhu J, Levi-D'Ancona E, Pearson GL, Reck EC, Walker EM, Kaufman BA, Soleimanpour SA
Nature communications 2022 Apr 29;13(1):2340
Nature communications 2022 Apr 29;13(1):2340
Phosphorylation of CREB at Serine 142 and 143 Is Essential for Visual Cortex Plasticity.
Pulimood NS, Contreras M, Pruitt ME, Tarasiewicz A, Medina AE
eNeuro 2021 Sep-Oct;8(5)
eNeuro 2021 Sep-Oct;8(5)
Proteomics reveal cap-dependent translation inhibitors remodel the translation machinery and translatome.
Ho JJD, Cunningham TA, Manara P, Coughlin CA, Arumov A, Roberts ER, Osteen A, Kumar P, Bilbao D, Krieger JR, Lee S, Schatz JH
Cell reports 2021 Oct 12;37(2):109806
Cell reports 2021 Oct 12;37(2):109806
Amelioration of Murine Macrophage Activation Syndrome by Monomethyl Fumarate in Both a Heme Oxygenase 1-Dependent and Heme Oxygenase 1-Independent Manner.
Biswas C, Chu N, Burn TN, Kreiger PA, Behrens EM
Arthritis & rheumatology (Hoboken, N.J.) 2021 May;73(5):885-895
Arthritis & rheumatology (Hoboken, N.J.) 2021 May;73(5):885-895
Structure of the full-length human Pannexin1 channel and insights into its role in pyroptosis.
Zhang S, Yuan B, Lam JH, Zhou J, Zhou X, Ramos-Mandujano G, Tian X, Liu Y, Han R, Li Y, Gao X, Li M, Yang M
Cell discovery 2021 May 4;7(1):30
Cell discovery 2021 May 4;7(1):30
TAPBPR promotes antigen loading on MHC-I molecules using a peptide trap.
McShan AC, Devlin CA, Morozov GI, Overall SA, Moschidi D, Akella N, Procko E, Sgourakis NG
Nature communications 2021 May 26;12(1):3174
Nature communications 2021 May 26;12(1):3174
Distinct states of proinsulin misfolding in MIDY.
Haataja L, Arunagiri A, Hassan A, Regan K, Tsai B, Dhayalan B, Weiss MA, Liu M, Arvan P
Cellular and molecular life sciences : CMLS 2021 Aug;78(16):6017-6031
Cellular and molecular life sciences : CMLS 2021 Aug;78(16):6017-6031
Tumor suppressive function of Matrin 3 in the basal-like breast cancer.
Yang J, Lee SJ, Kwon Y, Ma L, Kim J
Biological research 2020 Sep 25;53(1):42
Biological research 2020 Sep 25;53(1):42
Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication.
Ma-Lauer Y, Zheng Y, Malešević M, von Brunn B, Fischer G, von Brunn A
Antiviral research 2020 Jan;173:104620
Antiviral research 2020 Jan;173:104620
MAFG-driven astrocytes promote CNS inflammation.
Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, Rotem A, Heyman JA, Thaploo S, Sanmarco LM, Ragoussis J, Weitz DA, Petrecca K, Moffitt JR, Becher B, Antel JP, Prat A, Quintana FJ
Nature 2020 Feb;578(7796):593-599
Nature 2020 Feb;578(7796):593-599
Loss of tyrosine kinase receptor Ephb2 impairs proliferation and stem cell activity of spermatogonia in culture†.
N'Tumba-Byn T, Yamada M, Seandel M
Biology of reproduction 2020 Apr 15;102(4):950-962
Biology of reproduction 2020 Apr 15;102(4):950-962
Cyclophilin B control of lysine post-translational modifications of skin type I collagen.
Terajima M, Taga Y, Cabral WA, Liu Y, Nagasawa M, Sumida N, Kayashima Y, Chandrasekaran P, Han L, Maeda N, Perdivara I, Hattori S, Marini JC, Yamauchi M
PLoS genetics 2019 Jun;15(6):e1008196
PLoS genetics 2019 Jun;15(6):e1008196
TRIM5α Restricts Flavivirus Replication by Targeting the Viral Protease for Proteasomal Degradation.
Chiramel AI, Meyerson NR, McNally KL, Broeckel RM, Montoya VR, Méndez-Solís O, Robertson SJ, Sturdevant GL, Lubick KJ, Nair V, Youseff BH, Ireland RM, Bosio CM, Kim K, Luban J, Hirsch VM, Taylor RT, Bouamr F, Sawyer SL, Best SM
Cell reports 2019 Jun 11;27(11):3269-3283.e6
Cell reports 2019 Jun 11;27(11):3269-3283.e6
Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes β-catenin.
Kim J, Alavi Naini F, Sun Y, Ma L
American journal of cancer research 2018;8(9):1823-1836
American journal of cancer research 2018;8(9):1823-1836
Disruption of the β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner.
Mancini A, Xavier-Magalhães A, Woods WS, Nguyen KT, Amen AM, Hayes JL, Fellmann C, Gapinske M, McKinney AM, Hong C, Jones LE, Walsh KM, Bell RJA, Doudna JA, Costa BM, Song JS, Perez-Pinera P, Costello JF
Cancer cell 2018 Sep 10;34(3):513-528.e8
Cancer cell 2018 Sep 10;34(3):513-528.e8
Target-Based Screening against eIF4A1 Reveals the Marine Natural Product Elatol as a Novel Inhibitor of Translation Initiation with In Vivo Antitumor Activity.
Peters TL, Tillotson J, Yeomans AM, Wilmore S, Lemm E, Jiménez-Romero C, Amador LA, Li L, Amin AD, Pongtornpipat P, Zerio CJ, Ambrose AJ, Paine-Murrieta G, Greninger P, Vega F, Benes CH, Packham G, Rodríguez AD, Chapman E, Schatz JH
Clinical cancer research : an official journal of the American Association for Cancer Research 2018 Sep 1;24(17):4256-4270
Clinical cancer research : an official journal of the American Association for Cancer Research 2018 Sep 1;24(17):4256-4270
Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence.
Vera-Ramirez L, Vodnala SK, Nini R, Hunter KW, Green JE
Nature communications 2018 May 22;9(1):1944
Nature communications 2018 May 22;9(1):1944
Long noncoding RNA MALAT1 suppresses breast cancer metastasis.
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, Lee H, Zhou Z, Gan B, Nakagawa S, Ellis MJ, Liang H, Hung MC, You MJ, Sun Y, Ma L
Nature genetics 2018 Dec;50(12):1705-1715
Nature genetics 2018 Dec;50(12):1705-1715
The Role of CREB, SRF, and MEF2 in Activity-Dependent Neuronal Plasticity in the Visual Cortex.
Pulimood NS, Rodrigues WDS Junior, Atkinson DA, Mooney SM, Medina AE
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Jul 12;37(28):6628-6637
The Journal of neuroscience : the official journal of the Society for Neuroscience 2017 Jul 12;37(28):6628-6637
Expression of Peroxisome Proliferator-Activated Receptor alpha (PPARα) in somatotropinomas: Relationship with Aryl hydrocarbon receptor Interacting Protein (AIP) and in vitro effects of fenofibrate in GH3 cells.
Rotondi S, Modarelli A, Oliva MA, Rostomyan L, Sanita P, Ventura L, Daly AF, Esposito V, Angelucci A, Arcella A, Giangaspero F, Beckers A, Jaffrain-Rea ML
Molecular and cellular endocrinology 2016 May 5;426:61-72
Molecular and cellular endocrinology 2016 May 5;426:61-72
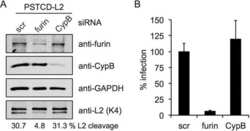
Furin Cleavage of L2 during Papillomavirus Infection: Minimal Dependence on Cyclophilins.
Bronnimann MP, Calton CM, Chiquette SF, Li S, Lu M, Chapman JA, Bratton KN, Schlegel AM, Campos SK
Journal of virology 2016 Jul 15;90(14):6224-6234
Journal of virology 2016 Jul 15;90(14):6224-6234
miR-100 induces epithelial-mesenchymal transition but suppresses tumorigenesis, migration and invasion.
Chen D, Sun Y, Yuan Y, Han Z, Zhang P, Zhang J, You MJ, Teruya-Feldstein J, Wang M, Gupta S, Hung MC, Liang H, Ma L
PLoS genetics 2014 Feb;10(2):e1004177
PLoS genetics 2014 Feb;10(2):e1004177
Thyroid hormone and estradiol have overlapping effects on kidney glutathione S-transferase-α gene expression.
Faustino LC, Almeida NA, Pereira GF, Ramos RG, Soares RM, Morales MM, Pazos-Moura CC, Ortiga-Carvalho TM
American journal of physiology. Endocrinology and metabolism 2012 Sep 15;303(6):E787-97
American journal of physiology. Endocrinology and metabolism 2012 Sep 15;303(6):E787-97
LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker.
Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, Ma L
Nature medicine 2012 Oct;18(10):1511-7
Nature medicine 2012 Oct;18(10):1511-7
Thyroid hormone contributes to the hypolipidemic effect of polyunsaturated fatty acids from fish oil: in vivo evidence for cross talking mechanisms.
Souza LL, Cordeiro A, Oliveira LS, de Paula GS, Faustino LC, Ortiga-Carvalho TM, Oliveira KJ, Pazos-Moura CC
The Journal of endocrinology 2011 Oct;211(1):65-72
The Journal of endocrinology 2011 Oct;211(1):65-72
Blocking leptin action one week after weaning reverts most of the programming caused by neonatal hyperleptinemia in the adult rat.
Trotta PA, Moura EG, Franco JG, Lima NS, de Oliveira E, Cordeiro A, Souza LL, Oliveira KJ, Lisboa PC, Pazos Moura CC, Passos MC
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2011 Mar;43(3):171-7
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2011 Mar;43(3):171-7
Protein kinase C activity regulates D-serine availability in the brain.
Vargas-Lopes C, Madeira C, Kahn SA, Albino do Couto I, Bado P, Houzel JC, De Miranda J, de Freitas MS, Ferreira ST, Panizzutti R
Journal of neurochemistry 2011 Jan;116(2):281-90
Journal of neurochemistry 2011 Jan;116(2):281-90
Activation of D1/D5 dopamine receptors protects neurons from synapse dysfunction induced by amyloid-beta oligomers.
Jürgensen S, Antonio LL, Mussi GE, Brito-Moreira J, Bomfim TR, De Felice FG, Garrido-Sanabria ER, Cavalheiro ÉA, Ferreira ST
The Journal of biological chemistry 2011 Feb 4;286(5):3270-6
The Journal of biological chemistry 2011 Feb 4;286(5):3270-6
Liver glutathione S-transferase expression is decreased by 3,5,3-triiodothyronine in hypothyroid but not in euthyroid mice.
Faustino LC, Pires RM, Lima AC, Cordeiro A, Souza LL, Ortiga-Carvalho TM
Experimental physiology 2011 Aug;96(8):790-800
Experimental physiology 2011 Aug;96(8):790-800
Small interfering RNA knocks down the molecular target of alendronate, farnesyl pyrophosphate synthase, in osteoclast and osteoblast cultures.
Wang Y, Panasiuk A, Grainger DW
Molecular pharmaceutics 2011 Aug 1;8(4):1016-24
Molecular pharmaceutics 2011 Aug 1;8(4):1016-24
Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation.
Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM
Nature cell biology 2011 Apr;13(4):383-93
Nature cell biology 2011 Apr;13(4):383-93
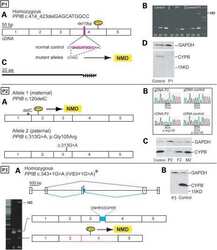
Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes.
Pyott SM, Schwarze U, Christiansen HE, Pepin MG, Leistritz DF, Dineen R, Harris C, Burton BK, Angle B, Kim K, Sussman MD, Weis M, Eyre DR, Russell DW, McCarthy KJ, Steiner RD, Byers PH
Human molecular genetics 2011 Apr 15;20(8):1595-609
Human molecular genetics 2011 Apr 15;20(8):1595-609
Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I.
Nouws J, Nijtmans L, Houten SM, van den Brand M, Huynen M, Venselaar H, Hoefs S, Gloerich J, Kronick J, Hutchin T, Willems P, Rodenburg R, Wanders R, van den Heuvel L, Smeitink J, Vogel RO
Cell metabolism 2010 Sep 8;12(3):283-94
Cell metabolism 2010 Sep 8;12(3):283-94
Cyclophilin B interacts with sodium-potassium ATPase and is required for pump activity in proximal tubule cells of the kidney.
Suñé G, Sarró E, Puigmulé M, López-Hellín J, Zufferey M, Pertel T, Luban J, Meseguer A
PloS one 2010 Nov 10;5(11):e13930
PloS one 2010 Nov 10;5(11):e13930
Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation.
Takahashi H, Wang Y, Grainger DW
Journal of controlled release : official journal of the Controlled Release Society 2010 Nov 1;147(3):400-7
Journal of controlled release : official journal of the Controlled Release Society 2010 Nov 1;147(3):400-7
siRNA knock-down of RANK signaling to control osteoclast-mediated bone resorption.
Wang Y, Grainger DW
Pharmaceutical research 2010 Jul;27(7):1273-84
Pharmaceutical research 2010 Jul;27(7):1273-84
Calcineurin signaling regulates human islet {beta}-cell survival.
Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, Stoffers DA
The Journal of biological chemistry 2010 Dec 17;285(51):40050-9
The Journal of biological chemistry 2010 Dec 17;285(51):40050-9
Amyloid-β triggers the release of neuronal hexokinase 1 from mitochondria.
Saraiva LM, Seixas da Silva GS, Galina A, da-Silva WS, Klein WL, Ferreira ST, De Felice FG
PloS one 2010 Dec 16;5(12):e15230
PloS one 2010 Dec 16;5(12):e15230
Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation.
Kenworthy R, Lambert D, Yang F, Wang N, Chen Z, Zhu H, Zhu F, Liu C, Li K, Tang H
Nucleic acids research 2009 Oct;37(19):6587-99
Nucleic acids research 2009 Oct;37(19):6587-99
Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF.
Ham JN, Crutchlow MF, Desai BM, Simmons RA, Stoffers DA
Pediatric research 2009 Jul;66(1):42-6
Pediatric research 2009 Jul;66(1):42-6
Soluble oligomers from a non-disease related protein mimic Abeta-induced tau hyperphosphorylation and neurodegeneration.
Vieira MN, Forny-Germano L, Saraiva LM, Sebollela A, Martinez AM, Houzel JC, De Felice FG, Ferreira ST
Journal of neurochemistry 2007 Oct;103(2):736-48
Journal of neurochemistry 2007 Oct;103(2):736-48
Characterization of hepatitis C virus subgenomic replicon resistance to cyclosporine in vitro.
Robida JM, Nelson HB, Liu Z, Tang H
Journal of virology 2007 Jun;81(11):5829-40
Journal of virology 2007 Jun;81(11):5829-40
Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion.
Agardh CD, Gustavsson C, Hagert P, Nilsson M, Agardh E
Metabolism: clinical and experimental 2006 Jul;55(7):892-8
Metabolism: clinical and experimental 2006 Jul;55(7):892-8
The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter.
Deramaudt TB, Sachdeva MM, Wescott MP, Chen Y, Stoffers DA, Rustgi AK
The Journal of biological chemistry 2006 Dec 15;281(50):38385-95
The Journal of biological chemistry 2006 Dec 15;281(50):38385-95
Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins.
Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, Dröse S, Brandt U, Hoffmann GF, Ter Laak H, Kölker S, Smeitink JA
The Biochemical journal 2006 Aug 15;398(1):107-12
The Biochemical journal 2006 Aug 15;398(1):107-12
Role of cyclophilin B in activation of interferon regulatory factor-3.
Obata Y, Yamamoto K, Miyazaki M, Shimotohno K, Kohno S, Matsuyama T
The Journal of biological chemistry 2005 May 6;280(18):18355-60
The Journal of biological chemistry 2005 May 6;280(18):18355-60
Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins.
Ling C, Poulsen P, Carlsson E, Ridderstråle M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A
The Journal of clinical investigation 2004 Nov;114(10):1518-26
The Journal of clinical investigation 2004 Nov;114(10):1518-26
The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system. Characterization of the system in normal and warfarin-resistant rats.
Wajih N, Sane DC, Hutson SM, Wallin R
The Journal of biological chemistry 2004 Jun 11;279(24):25276-83
The Journal of biological chemistry 2004 Jun 11;279(24):25276-83
High binding capacity of cyclophilin B to chondrocyte heparan sulfate proteoglycans and its release from the cell surface by matrix metalloproteinases: possible role as a proinflammatory mediator in arthritis.
De Ceuninck F, Allain F, Caliez A, Spik G, Vanhoutte PM
Arthritis and rheumatism 2003 Aug;48(8):2197-206
Arthritis and rheumatism 2003 Aug;48(8):2197-206
Transport activity and surface expression of the Na+-Ca2+ exchanger NCX1 are inhibited by the immunosuppressive agent cyclosporin A and by the nonimmunosuppressive agent PSC833.
Kimchi-Sarfaty C, Kasir J, Ambudkar SV, Rahamimoff H
The Journal of biological chemistry 2002 Jan 25;277(4):2505-10
The Journal of biological chemistry 2002 Jan 25;277(4):2505-10
Selective intracellular retention of extracellular matrix proteins and chaperones associated with pseudoachondroplasia.
Vranka J, Mokashi A, Keene DR, Tufa S, Corson G, Sussman M, Horton WA, Maddox K, Sakai L, Bächinger HP
Matrix biology : journal of the International Society for Matrix Biology 2001 Nov;20(7):439-50
Matrix biology : journal of the International Society for Matrix Biology 2001 Nov;20(7):439-50
Selective intracellular retention of extracellular matrix proteins and chaperones associated with pseudoachondroplasia.
Vranka J, Mokashi A, Keene DR, Tufa S, Corson G, Sussman M, Horton WA, Maddox K, Sakai L, Bächinger HP
Matrix biology : journal of the International Society for Matrix Biology 2001 Nov;20(7):439-50
Matrix biology : journal of the International Society for Matrix Biology 2001 Nov;20(7):439-50
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
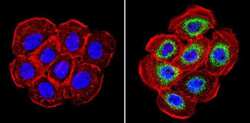
- Experimental details
- Immunofluorescent analysis of Cyclophilin B (green) showing staining in the in the cytoplasm of A431 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) in 3% BSA-PBS at a dilution of 1:500 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
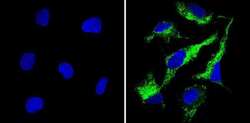
- Experimental details
- Immunofluorescent analysis of Cyclophilin B (green) showing staining in the in the cytoplasm of HepG2 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) in 3% BSA-PBS at a dilution of 1:200 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
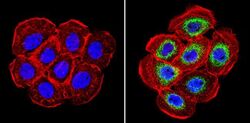
- Experimental details
- Immunofluorescent analysis of Cyclophilin B (green) showing staining in the in the cytoplasm of A431 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) in 3% BSA-PBS at a dilution of 1:500 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
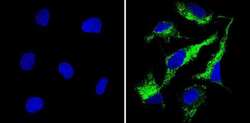
- Experimental details
- Immunofluorescent analysis of Cyclophilin B (green) showing staining in the in the cytoplasm of HepG2 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) in 3% BSA-PBS at a dilution of 1:200 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
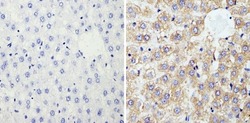
- Experimental details
- Immunohistochemistry analysis of Cyclophilin B showing staining in the cytoplasm of paraffin-embedded rat liver tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
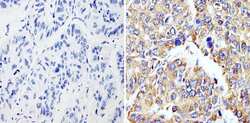
- Experimental details
- Immunohistochemistry analysis of Cyclophilin B showing staining in the cytoplasm of paraffin-embedded human hepatocarcinoma (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) diluted in 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
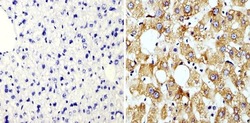
- Experimental details
- Immunohistochemistry analysis of Cyclophilin B showing staining in the cytoplasm of paraffin-embedded human liver tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclophilin B polyclonal antibody (Product # PA1-027A) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
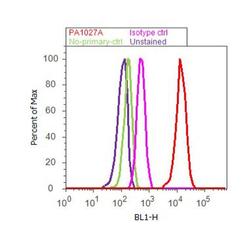
- Experimental details
- Flow cytometry analysis of Cyclophilin B was done on U-87 MG cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Cyclophilin B Rabbit Polyclonal Antibody (PA1027A, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control..
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
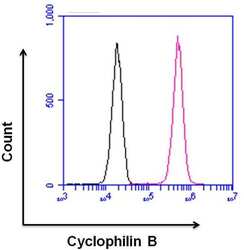
- Experimental details
- Flow cytometry analysis of Cyclophilin B was done on HeLa cells. Cells were fixed, permeabilized and stained with a Cyclophilin B rabbit polyclonal antibody (Product # PA1-027A, pink histogram) or a rabbit IgG isotype control (Product # MA5-16384, black histogram) at a dilution of 10 µg/mL. After incubation for 1 hour on ice, the cells were labeled with a Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 647 conjugate (Product # A27040) at a dilution of 1:50 for 1 hour on ice. A representative 10,000 cells were acquired and analyzed for each sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
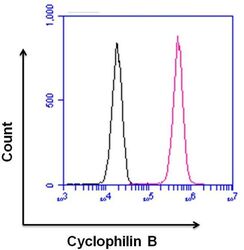
- Experimental details
- Flow cytometry analysis of Cyclophilin B was done on HeLa cells. Cells were fixed, permeabilized and stained with a Cyclophilin B rabbit polyclonal antibody (Product # PA1-027A, pink histogram) or a rabbit IgG isotype control (Product # MA5-16384, black histogram) at a dilution of 10 µg/mL. After incubation for 1 hour on ice, the cells were labeled with a Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 647 conjugate (Product # A27040) at a dilution of 1:50 for 1 hour on ice. A representative 10,000 cells were acquired and analyzed for each sample.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
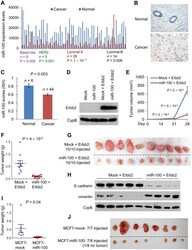
- Figure 2 miR-100 inhibits tumorigenesis and is downregulated in human breast cancer. (A) miR-100 expression levels in four subtypes of human breast tumors and paired normal breast tissues, based on the RNA-Seq data from TCGA. Statistical significance was determined by paired t test. (B) Representative images of in situ hybridization of miR-100 in normal human mammary glands and human breast carcinomas. Blue color indicates positive staining. (C) miR-100 scores (normalized to U6) based on in situ hybridization of human breast tissue microarray (TMA). (D) Immunoblotting of Erbb2 and cyclophilin B (CypB) in HMLE cells transduced with miR-100 and Erbb2, alone or in combination. (E) Tumor growth by 1.5x10 6 subcutaneously injected HMLE cells transduced with Erbb2 alone or in combination with miR-100. Data are mean +- SEM ( n = 10 mice per group). (F, G) Tumor weight (F) and tumor images (G) of mice with subcutaneous injection of HMLE cells transduced with Erbb2 alone or in combination with miR-100, at day 31 after implantation. Data in (F) are mean +- SEM ( n = 10 mice per group). (H) Immunoblotting of E-cadherin, vimentin and cyclophilin B (CypB) in tumor lysates from (G). (I, J) Tumor weight (I) and tumor images (J) of mice with subcutaneous injection of 5x10 6 miR-100-transduced MCF7 cells, at day 22 after implantation. Data in (I) are mean +- SEM ( n = 7-8 mice per group). Statistical significance in (C), (E), (F) and (I) was determined by two-tailed, unpaired Student's t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
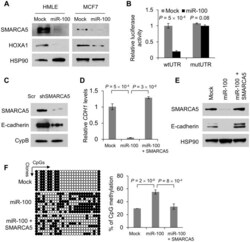
- Figure 3 miR-100 downregulates E-cadherin by targeting SMARCA5 . (A) Immunoblotting of SMARCA5, HOXA1 and HSP90 in HMLE and MCF7 cells transduced with miR-100. (B) Luciferase activity of the wild-type or mutant HOXA1 3' UTR reporter gene in 293T cells with ectopic expression of miR-100. (C) Immunoblotting of SMARCA5, E-cadherin and cyclophilin B (CypB) in HMLE cells infected with the SMARCA5 shRNA (shSMARCA5) or the pLKO.1-puro lentiviral vector with a scrambled sequence (Scr) that does not target any mRNA. (D) qPCR of CDH1 in HMLE cells transduced with the control vector (mock), miR-100 alone or in combination with SMARCA5. (E) Immunoblotting of SMARCA5, E-cadherin and HSP90 in HMLE cells transduced with the control vector (mock), miR-100 alone or in combination with SMARCA5. (F) Bisulfite sequencing assay (left panel) and the percentage of CpG methylation (right panel) of the CDH1 promoter in HMLE cells transduced with the control vector (mock), miR-100 alone or in combination with SMARCA5. Open circles: unmethylated CpG sites; solid black circles: methylated CpG sites. Data in (B), (D) and (F) are mean +- SEM, and statistical significance was determined by two-tailed, unpaired Student's t test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
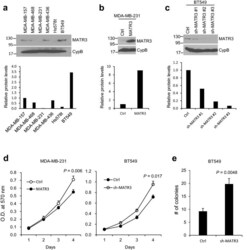
- Fig. 1 Comparison of MATR3 protein levels in a panel of basal-like/triple-negative human breast cancer cell lines. a Endogenous MATR3 protein levels were examined in a small panel of basal-like/triple-negative breast cancer cell lines by western blotting. The lower panel demonstrates the relative normalized MATR3 protein levels. b MATR3 was transiently overexpressed in MDA-MB-231 cells and the expression was determined by western blotting. The lower panel demonstrates the relative normalized MATR3 protein levels. c MATR3 was silenced by shRNAs and the expression was determined by western blotting. The lower panel demonstrates the relative normalized MATR3 protein levels. d In vitro cell proliferation was compared between control and MATR3-overexpressing MDA-MB-231 and between control and MATR3-depleted BT549 cells. n = 4 per group. e Anchorage-independent growth was examined to compare in vitro tumorigenesis by soft agar colony formation assay. n = 4 per group. Statistical significance in d and e was determined by an unpaired t -test. Error bars are s.e.m
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5. MALAT1 inactivates TEAD ( a ) Luciferase activity in HEK293FT cells co-transfected with indicated plasmids. n = 4 cell culture replicates per group. ( b ) Luciferase activity in control and MALAT1 knockout MDA-MB-231 cells co-transfected with indicated plasmids. n = 3 cell culture replicates per group. ( c, d ) HEK293FT cells were co-transfected with Malat1 , SFB-YAP, and HA-TEAD1, and were subjected to pulldown with S-protein beads ( c ) or an HA-specific antibody ( d ), followed by immunoblotting with antibodies against pan-TEAD and FLAG. ( e ) ChIP-qPCR analysis showing the occupancy of ANKRD1 , CTGF , and CYR61 promoters by TEAD1 or YAP immunoprecipitated from control or MALAT1 knockout MDA-MB-231 cells. ( f ) qPCR of YAP-TEAD target genes in the tumors of MMTV-PyMT; Malat1 +/+ (PyMT;WT), MMTV-PyMT; Malat1 -/- (PyMT;KO), and MMTV-PyMT; Malat1 -/- ; Malat1 Tg (PyMT;KO;Tg) mice. n = 5 mice per group. ( g ) Immunoblotting of pan-TEAD and cyclophilin B (CypB) in MALAT1 knockout MDA-MB-231 cells with or without transduction of TEAD shRNA. Scr: scramble control. ( h, i ) Bioluminescent imaging ( h ) and photon flux quantification ( i ) of NSG mice with intravenous injection of control and MALAT1 knockout MDA-MB-231 cells with or without transduction of TEAD shRNA. Day 0: the day of tumor cell injection. n = 5 mice per group. ( j ) Bioluminescent imaging (upper panel) and photon flux quantification (lower panel) of the lungs from mice described in Fig. 5h, i . n = 5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
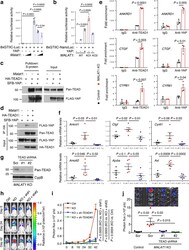
- Fig. 2 Important chaperone features for functional replacement of tapasin localize to scaffolding sites for MHC-I. a TAPBPR and variants of tapasin were transfected in tapasin-KO Expi293F cells, and relative surface HLA-A*02:01 expression as measured by flow cytometry is plotted. Data are mean +- SD, n = 4 independent experiments. Proteins were FLAG-tagged at their luminal N-termini and immunoblots of the whole lysate are shown, with cyclophilin B (CycB) used as a loading control. b Tapasin was deep mutationally scanned based on selection of tapasin-KO cells with rescued surface HLA-A*02:01. Log 2 enrichment ratios for mutations across residues 10-20 are plotted from =+3 (enriched, dark blue). Residue position is on the horizontal axis, and amino acid substitutions are on the vertical axis (*, stop codon). c Conservation scores from the entire deep mutational scan of the tapasin/MHC-I interface are mapped to a homology model of HLA-A*02:01-bound tapasin. Highly conserved residues for mediating the folding and surface trafficking of HLA-A*02:01 are colored orange, while neutral regions are pale white/blue. Residues excluded from the library and analysis are gray. MHC-I H chain and hbeta 2 m are pale and dark green ribbons, respectively. d A chimera of the TAPBPR luminal domain with the tapasin TM and cytosolic domains, called TAPBPR-CT, has increased activity for chaperoning endogenous HLA-A*02:01, despite reduced protein expression by i
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 2. Rocaglate-inducible GEF-H1 regulates JNK signaling via RHOA (A) Schematic of RHOA/JNK activation by rocaglate-induced GEF-H1 expression. (B) Relative total cell number of U87MG treated with indicated siRNAs (pool of four independent siRNAs for each target) for 48 h, followed by silvestrol treatment at indicated concentrations or vehicle (DMSO) for 24 h. Data represent mean +- SEM (error bars) of three independent experiments. *p < 0.05 compared to NS (non-silencing) siRNA control. (C) Representative immunoblots of U87MG treated with indicated concentrations of silvestrol, rocaglamide A, or vehicle (DMSO) for 24 h. (D) Representative immunoblots of U87MG treated with indicated siRNA pools for 48 h, followed by silvestrol treatment at indicated concentrations or vehicle (DMSO) for 24 h. (E) Representative immunoblots of U87MG treated with indicated 3' UTR-targeting siRNA pools and expression construct simultaneously for 48 h, followed by treatment with silvestrol (6.25 nM) or vehicle (DMSO) for 24 h. GEF-H1 ORF, coding region-only construct; short, short exposure; long, long exposure. (F) Representative immunoblots of U87MG treated with indicated siRNA pools (100 nM final concentration) for 72 h, followed by treatment with silvestrol (12.5 nM) or vehicle (DMSO) for 24 h. Densitometry values in (C) and (D) are mean of three independent experiments, (E) and (F) are normalized to loading control. (G) Representative immunoblots of active RHO (RHO-GTP) pull-down lysates fr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
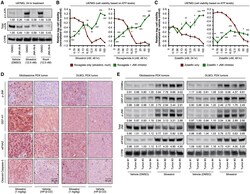
- Figure 3. Rocaglate-inducible JNK phosphorylation mediates cellular toxicity signaling (A) Representative immunoblots of U87MG treated with silvestrol (12.5 nM), rocaglamide A (rocA, 12.5 nM), or vehicle (DMSO) with and without JNK inhibitor JNK-IN-8 (10 muM) for 24 h. Densitometry values (mean of three independent experiments) are normalized to loading control. (B and C) Relative cell viability based on ATP levels in U87MG treated with indicated concentrations of (B) silvestrol (left panel) and rocaglamide A (right panel) or (C) zotatifin with and without JNK inhibitor JNK-IN-8 (10 muM) for the indicated durations. For (B) and (C), data represent mean +- SEM (error bars) of four independent cell populations. *p < 0.05, **p < 0.01, ***p < 0.001 compared to corresponding rocaglate only condition. (D) Representative immunohistochemistry images (D; scale bars represent 50 mum) and immunoblots (E) of glioblastoma (left panel) and DLBCL (right panel) patient-derived xenograft mouse tumors treated with silvestrol (1 mg/kg) or vehicle (2-hydroxypropyl-beta-cyclodextrin) (daily intraperitoneal injection for 3 days). For (E), densitometry values are normalized to loading control. See also Figure S3 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
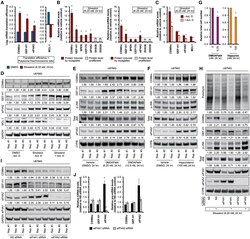
- Figure 4. Mechanisms of rocaglate-dependent protein induction (A) mRNA translation efficiency (abundance ratio of polysome-associated mRNA to ribosome-free and monosome-associated mRNA) in U87MG treated with silvestrol (6.25 nM) for 24 h. Data represent mean +- SEM (error bars) of five independent experiments. *p < 0.05 compared to vehicle (DMSO) treatment. (B) Steady-state mature mRNA (left panel) and pre-mRNA (right panel) levels in U87MG treated with silvestrol (6.25 nM) for 24 h. Data represent mean +- SEM (error bars) of five independent experiments. *p < 0.05 compared to vehicle (DMSO) treatment. Data are normalized against 18S rRNA. (C and D) Steady-state mRNA levels (C) and representative immunoblots (D) of U87MG treated with silvestrol (6.25 nM) for 24 h with and without actinomycin D (Act. D, 1 mug/mL, for the last 6 h of silvestrol treatment). Densitometry values in (D) are normalized to loading control. Data in (C) represent mean +- SEM (error bars) of three independent experiments. *p < 0.05 compared to no Act. D treatment. (E and F) Representative immunoblots of U87MG treated with (E) DMDAPatA (indicated concentrations), (F) hippuristanol (100 nM), or vehicle (DMSO) for 24 h. Densitometry values in (E) and (F) are normalized to loading control. (G) Relative total cell number of U87MG treated with DMDAPatA (6.25 nM) (left panel), hippuristanol (100 nM) (right panel), or vehicle (DMSO) for 24 h. Data represent mean +- SEM (error bars) of three independent experime
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
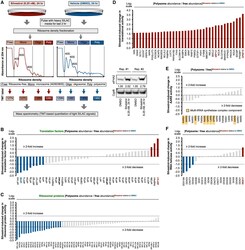
- Figure 5. System-wide survey of rocaglate-dependent changes in the translation machinery (A) MATRIX workflow of silvestrol treatment in U87MG. Polysome fractions reflect productive, high-intensity protein synthesis, whereas ribosome-free fractions are associated with translational inactivity. Representative immunoblots of U87MG input samples for ribosome density fractionation, lysed with polysome lysis buffer, are shown at the bottom right. (B-F) MATRIX analysis of silvestrol-induced changes in translational activity (i.e., ratio of protein abundance in polysome versus ribosome-free [free] fraction) for (B) canonical translation factors, (C) ribosomal proteins, (D) most highly induced proteins (>=3-fold activity enrichment), (E) aminoacyl-tRNA synthetases (AARSs), and (F) DEAD/DEAH RNA helicases. Blue and red indicate silvestrol-repressed and silvestrol-activated translational assets (>=2-fold difference in activity), respectively. See also Figure S5 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
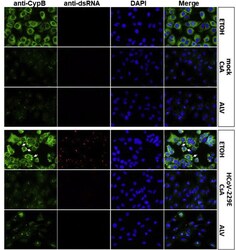
- Fig. 5 Immunostaining of CypB in mock- and HCoV-229E-infected Huh7 cells in the presence of ETOH solvent or cyclophilin inhibitors CsA and Alisporivir . After infection (MOI = 1) medium was removed and new medium was added to cells containing 20 muM of inhibitor for 48 h and samples were processed for IF. CypB and dsRNA were stained with anti-CypB (green) and anti-dsRNA J2 (red, Scicons, 1:1500), respectively. Nuclei were visualized with DAPI. CypB shifts from an even cytoplasmic distribution to intense bleb-like structures in the presence of virus (white arrows, ETOH solvent panels). CsA and ALV led to the re-localization of CypB to granular structures in the nucleus and to a massive reduction of expression independent of virus infection. dsRNA as replication marker was not detected in the presence of CsA or ALV. Exposure times for the respective antibodies were the same in the different samples. Fig. 5
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
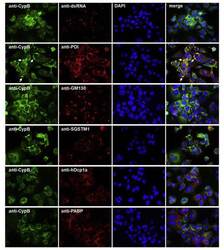
- Fig. 6 Co-immunostaining of CypB and cell organelles in HCoV-229E-infected Huh7 cells . For the identification of the intense cyclophilin B bleb-like structures infected cells (MOI = 1; 48 h p.i.) were co-stained with anti-CypB and antibodies directed against markers of the ER (anti-PDI), cis-GOLGI (anti-GM130), autophagosomes (anti-SQSTM1), anti-P-bodies (anti-hDcp1a) and stress granules (anti-PABP). Cyclophilin B normally distributes within the ER. In the presence of HCoV-229E, it intriguingly concentrates at bleb-like structures of the ER. Fig. 6
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
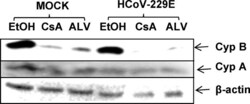
- Fig. 7 Downregulation of Cyp B, but not CypA in the presence of Cyp inhibitors CsA and ALV. Huh7 cells were either mock or HCoV-229E (MOI = 1) infected and cultivated in the presence of EtOH solvent, or 20 muM CsA or ALV for 48 h. Cell extracts were subjected to Western blot analysis and staining with anti-Cyp A, anti-Cyp B and anti-beta actin as loading control. Fig. 7
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
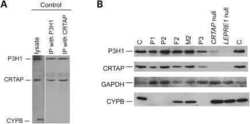
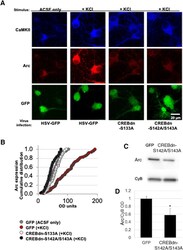
- Figure 3. Activity-dependent Arc expression is blocked in the absence of CREB phosphorylation at Ser133 as well as at Ser142/143. A , Stimulus and virus infection conditions are as follows (from left): ACSF control media in cells infected with HSV-GFP control virus ( n = 105 cells on 12 coverslips from 3 independent experiments), KCl-rich ACSF to depolarize cells infected with HSV-GFP control virus ( n = 128 cells on 15 coverslips from 3 independent experiments), KCl-rich ACSF in cells infected with CREBdn-S133A ( n = 51 cells on 20 coverslips from 3 independent experiments), and KCl-rich ACSF in cells infected with CREBdn-S142A/S143A ( n = 63 cells on 15 coverslips from 3 independent experiments). Top panels show Arc staining in red and bottom panels show HSV infection in green. B , A cumulative distribution plot showing all analyzed cells in each condition clearly displays the activity-dependent increase in Arc expression that is blocked by the CREBdn viruses [one-way ANOVA, F (3,333) = 53.1, p < 0.0001; Tukey's post hoc test GFP(+KCl) vs all other groups p < 0.0001]. C , Representative case of Arc expression in mice injected with CREBdn-S142A/S143A or control GFP. Cyclophilin B (CyB) was used as a loading control. D , Quantification shows a significant reduction in Arc expression after blocked phosphorylation of CREB at Ser142/143 ( t = 2.32, * p = 0.04; df = 8, t test for unequal variances). All error bars indicate standard error of the mean (SEM). OD = optical density.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
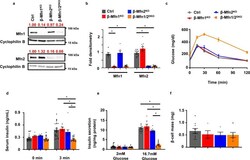
- Loss of both Mfn1 and Mfn2 in beta-cells impairs glucose tolerance and glucose-stimulated insulin release. a Expression of Mfn1 and Mfn2 by Western blot (WB) in islets isolated from 8-10-week-old Ctrl, beta-Mfn1 KO , beta-Mfn2 KO and beta-Mfn1/2 DKO mice. Cyclophilin B serves as a loading control. Representative of six independent mice/group for Mfn1 and 10 independent mice/group for Mfn2. Band intensity of each lane of displayed representative blot (shown as fold change compared to Ctrl, expression normalized to cyclophilin B) above each blot in red. b Densitometry (normalized to cyclophilin B) from studies in Fig. 1A. n = 6/group for Mfn1 and 10/group for Mfn2. Data are presented as Mean +- SEM. * p < 0.0005 by one-way ANOVA followed by Tukey's post-hoc test for multiple comparisons. c Blood glucose concentrations measured during IPGTT of 8-week-old Ctrl ( n = 28), beta-Mfn1 KO ( n = 7), beta-Mfn2 KO ( n = 6) and beta-Mfn1/2 DKO ( n = 15) littermates. Data are presented as Mean +- SEM. ( p < 0.0005 by two-way ANOVA for beta-Mfn1/2 DKO mice vs Ctrl followed by Sidak's post-hoc test for multiple comparisons). d Serum insulin concentrations ( n = 7-24/group) measured during in vivo glucose-stimulated insulin release testing in 8-week-old Ctrl ( n = 24), beta-Mfn1 KO ( n = 7), beta-Mfn2 KO ( n = 10) and beta-Mfn1/2 DKO ( n = 14) littermates. Data are presented as mean +- SEM. * p < 0.0005 for beta-Mfn1/2 DKO mice vs Ctrl and beta-Mfn1 KO , p = 0.004 for beta-Mfn1/2 KO vs beta-M
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
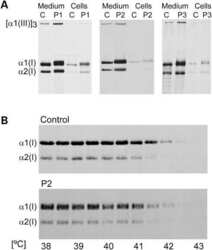
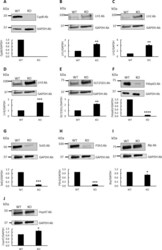
- Fig 1 Western blot analysis for lysine modifying enzymes and chaperone complex components in skin obtained from wild type (WT) and CypB KO (KO) mice. The protein levels in WT and KO were assessed by their immunoreactivities with the respective antibodies (Ab) relative to that of GAPDH. (A) CypB, (B) LH1, (C) LH2, (D) LH3, (E) GLT25D1, (F) Fkbp65, (G) Sc65, (H) P3h3, (I) Bip, and (J) Hsp47. *p
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry