PA5-23381
antibody from Invitrogen Antibodies
Targeting: STING1
ERIS, FLJ38577, MITA, MPYS, NET23, STING, TMEM173
Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [2]
- Flow cytometry [3]
- Other assay [3]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-23381 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- STING Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Sequence homology: Opossum/Zebrafish (83%), Xenopus (72%), Rat (88%).
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1.0 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Human rhinovirus promotes STING trafficking to replication organelles to promote viral replication.
ASA404, a vascular disrupting agent, as an experimental treatment approach for brain tumors.
AIM2-Like Receptors Positively and Negatively Regulate the Interferon Response Induced by Cytosolic DNA.
Triantafilou M, Ramanjulu J, Booty LM, Jimenez-Duran G, Keles H, Saunders K, Nevins N, Koppe E, Modis LK, Pesiridis GS, Bertin J, Triantafilou K
Nature communications 2022 Mar 17;13(1):1406
Nature communications 2022 Mar 17;13(1):1406
ASA404, a vascular disrupting agent, as an experimental treatment approach for brain tumors.
Bähr O, Gross S, Harter PN, Kirches E, Mawrin C, Steinbach JP, Mittelbronn M
Oncology letters 2017 Nov;14(5):5443-5451
Oncology letters 2017 Nov;14(5):5443-5451
AIM2-Like Receptors Positively and Negatively Regulate the Interferon Response Induced by Cytosolic DNA.
Nakaya Y, Lilue J, Stavrou S, Moran EA, Ross SR
mBio 2017 Jul 5;8(4)
mBio 2017 Jul 5;8(4)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
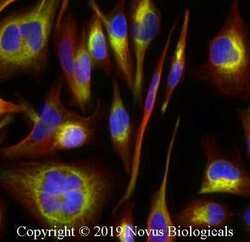
- Experimental details
- Immunocytochemistry analysis of STING in RH-30 cells fixed for 10 minutes using 10% formalin and then permeabilized for 5 minutes using 1X PBS + 0.05% Triton X-100. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 2 µg/mL overnight at 4 °C followed by anti-rabbit DyLight 488 (Green) with a dilution of 1:500. Alpha Tubulin Antibody (DM1A) was used as a co-stain at a 1:1000 dilution and detected with an anti-mouse DyLight 550 (Red) at a 1:500 dilution. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 40X objective.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
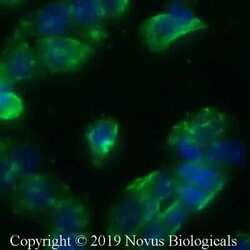
- Experimental details
- Immunocytochemistry analysis of STING in HT-29 cells fixed for 10 minutes using 10% formalin and then permeabilized for 5 minutes using 1X PBS + 0.05% Triton-X100. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 10 µg/mL overnight at 4 °C followed by anti-rabbit DyLight 488 (Green) with a dilution of 1:500. Nuclei were counterstained with DAPI (Blue). Cells were imaged using a 40X objective.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
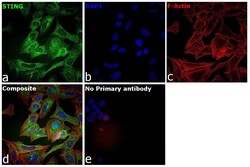
- Experimental details
- Immunofluorescence analysis of TMEM173 was performed using 70% confluent log phase U-2 OS cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.01% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with TMEM173 (Product # PA5-23381 ) at 1:100 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with Hoechst 33342 (Product # H1399). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image predominantly showing endoplasmic reticulum localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 40X magnification in CellInsight CX7 LZR High-Content Screening (HCS) Platform (Product # CX7C1115LZR). magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
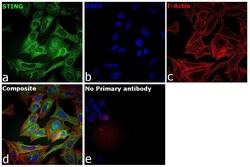
- Experimental details
- Immunofluorescence analysis of TMEM173 was performed using 70% confluent log phase U-2 OS cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.01% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with TMEM173 (Product # PA5-23381 ) at 1:100 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with Hoechst 33342 (Product # H1399). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image predominantly showing endoplasmic reticulum localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 40X magnification in CellInsight CX7 LZR High-Content Screening (HCS) Platform (Product # CX7C1115LZR). magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
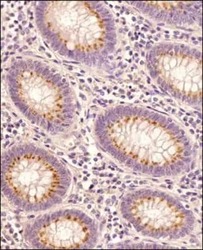
- Experimental details
- Immunohistochemical analysis of STING in Human colon cancer tissue section. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 1:100 followed by HRP-conjugated secondary antibody. The signal was developed using DAB reagent and the nuclei were counterstained with hematoxylin. The antibody generated very weak cytoplasmic staining in columnar epithelial cells with a very strong signal in the secretory/goblet cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
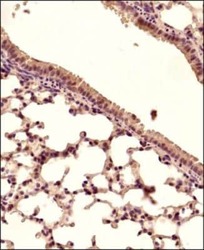
- Experimental details
- Immunohistochemical analysis of STING in Mouse lung tissue section. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 1:150 followed by a HRP-conjugated secondary antibody. The signal was developed using DAB reagent and the nuclei were counterstained with hematoxylin. The antibody generated mainly a cytoplasmic staining in the bronchiolar and alveolar epithelial cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
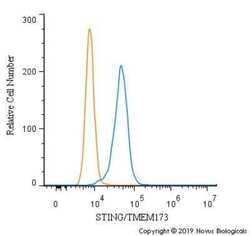
- Experimental details
- Flow cytometry of STING in RH30 cells (blue) and a matched isotype control (orange). Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 2.5 µg/mL for 30 minutes at room temperature followed by a Rabbit IgG (H+L) Cross-Adsorbed secondary antibody. Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
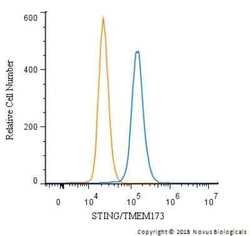
- Experimental details
- Flow cytometry of STING in THP-1 cells and a matched isotype control. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 2.5 µg/mL for 30 minutes at room temperature followed by a Rabbit IgG (H+L) Cross-Adsorbed secondary antibody. Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
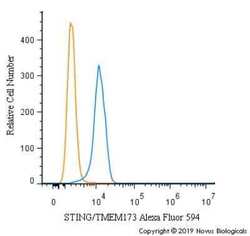
- Experimental details
- Flow cytometry of STING in U937 cells. Samples were incubated in STING polyclonal antibody (Product # PA5-23381) using a dilution of 2.5 µg/mL for 30 minutes at room temperature. Antibody (blue) and a matched isotype control (orange). Cells were fixed with 4% PFA and then permeabilized with 0.1% saponin. Both antibodies were conjugated to Alexa Fluor 594.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
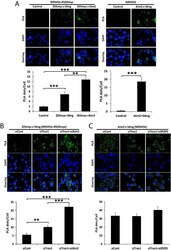
- Experimental details
- FIG 7 IFI205-STING interaction increases upon TREX1 and AIM2 depletion. (A) PLA for interactions of IFI205myc-AIM2, IFI205myc-STING, and AIM2-STING in NR9456-IFI205myc or NR9456 cells. The number of cells and images quantified for each condition in NR9456-IFI205myc cells are as follows: IFI205myc-Sting, 141 cells and 10 images; IFI205myc-Aim2, 101 cells and 6 images; control, 65 cells and 5 images. The number of cells and images quantified for each condition in NR9456 cells are as follows: control, 74 cells and 5 images; Aim2-Sting, 47 cells and 5 images. Controls for knockdowns are shown in Fig. S9A . (B) PLA for IFI205myc-STING interactions in NR9456-IFI205myc cells with knockdown of the genes indicated. Quantification: siCont, 102 cells and 5 images; siTrex1, 190 cells and 7 images; siTrex1+siAim2, 108 cells and 5 images. A representative image is shown. This experiment was repeated twice and gave similar results both times. (C) PLA for AIM2-STING interaction in NR9456 cells after knockdown of the genes indicated. Quantification: siCont, 84 cells and 4 images; siTrex1, 33 cells and 4 images; siTrex1+si205, 58 cells and 4 images. PLA dots were quantified and normalized to cell numbers based on DAPI staining with ImageJ software. The values shown are the mean +- the standard error of the mean of different pictures. **, P < 0.005; ***, P < 0.0005. (two-tailed t test). siCont, control siRNA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
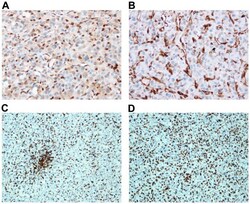
- Experimental details
- Figure 8. STING expression and iba1 positive cells in subcutaneous and intracranial U-87 tumors. Immunohistochemistry for STING is shown for subcutaneous (A) and intracranial (B) U-87 tumors. Cells positive for iba1 are stained in subcutaneous (C) and intracranial (D) U-87 tumors.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
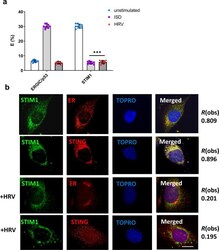
- Experimental details
- Fig. 5 STIM1 releases STING upon HRV infection. FRET studies measuring donor (STING) and acceptor (ERGIC or STIM1) interactions in BEAS-2B cells infected with HRV for 2 h or exposed to ISD (1 ug) ( a ). Confocal microscopy of BEAS-2B cells infected with HRV for 2 h assessing colocalization between STIM1, the ER and STING. Data in a is means +/- SD ( n = 3, independent experiments). Statistical significance between unstimulated and virus-infected conditions was assessed by unpaired student's t -test. *** p < 0.001 Data in b is representative of three biological donors with at least 20 technical replicates. Degree of colocalization is presented at R(obs). Bars shown at 10 um.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry