AHO1552
antibody from Invitrogen Antibodies
Targeting: TGFBR1
ACVRLK4, ALK-5, ALK5, ESS1, MSSE, TBR-i, TBRI
Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- AHO1552 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- TGFBR1 Monoclonal Antibody (234R32)
- Antibody type
- Monoclonal
- Antigen
- Recombinant protein fragment
- Reactivity
- Human, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 234R32
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references OTUD4 enhances TGFβ signalling through regulation of the TGFβ receptor complex.
Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway.
CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro.
Jaynes PW, Iyengar PV, Lui SKL, Tan TZ, Vasilevski N, Wright SCE, Verdile G, Jeyasekharan AD, Eichhorn PJA
Scientific reports 2020 Sep 24;10(1):15725
Scientific reports 2020 Sep 24;10(1):15725
Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway.
Guan R, Wang J, Cai Z, Li Z, Wang L, Li Y, Xu J, Li D, Yao H, Liu W, Deng B, Lu W
Redox biology 2020 Jan;28:101356
Redox biology 2020 Jan;28:101356
CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro.
Zhou S, da Silva SD, Siegel PM, Philip A
Scientific reports 2019 Nov 6;9(1):16317
Scientific reports 2019 Nov 6;9(1):16317
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
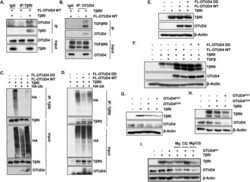
- Figure 3 OTUD4 regulates the ubiquitination of the TbetaR complex. ( A ) HEK293T cells were transfected with TbetaRI and/or FLAG-OTUD4. After 48 h cells were lysed and immunoprecipitated with anti- TbetaRI affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( B ) HEK293T cells were transfected with TbetaRII and FLAG-OTUD4. After 48 h cells were lysed and immunoprecipitated with anti- OTUD4 affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( C ) HEK293T cells were transfected with HA-ubiquitin, TbetaRI and either FLAG-OTUD4, or FLAG-OTUD4 DD. After 48 h cells were lysed and immunoprecipitated with anti- TbetaRI affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( D ) HEK293T cells were transfected with HA-ubiquitin, TbetaRII and either FLAG-OTUD4, or FLAG-OTUD4 DD. After 48 h cells were lysed and immunoprecipitated with anti-TbetaRII affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( E ) HEK293T cells were transfected with TbetaRI and either FLAG-OTUD4 or FLAG OTUD4 DD. Whole cell extracts were probed with indicated antibodies. beta-Actin is used as the loading control. ( F ) HEK293T cells were transfected with TbetaRII and either FLAG-OTUD4 or FLAG OTUD4 DD. Cells were stimulated where indicated with TGFbeta (100 pM) overnight before lysis. W
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
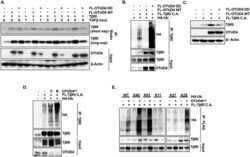
- Figure 4 OTUD4 regulates TbetaRI presence at the plasma membrane. ( A ) HEK293T cells were transfected with TbetaRI and either FLAG-OTUD4 or FLAG-OTUD4 DD. Cells were stimulated with TGFbeta (100 pM) for the length of time indicated. Cell surface was labelled with biotin at 4 degrees for 40 min before lysis. Lysates were immunoprecipitated with anti- NeutrAvidin affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. beta-Actin is used as the loading control. ( B ) HEK293T cells were transfected with HA-ubiquitin, FLAG-TbetaRI C.A. and either FLAG-OTUD4 or FLAG-OTUD4 DD. Cells were incubated with MG132 (5 uM) overnight before lysis. After 48 h cells were lysed and immunoprecipitated with anti-TbetaRI affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( C ) HEK293T cells were transfected with FLAG-TbetaRI C.A. and either FLAG-OTUD4 or FLAG OTUD4 DD. Whole cell extracts were probed with indicated antibodies. beta-Actin is used as the loading control. ( D ) HEK293T cells were transfected with HA-ubiquitin, FLAG-TbetaRI C.A. and OTUD4 shRNA constructs B or C. Cells were incubated with MG132 (5 uM) overnight before lysis. After 72 h cells were lysed and immunoprecipitated with anti-TbetaRI affinity resin. Immunoprecipitated lysates and whole cell extracts were probed with the indicated antibodies. ( E ) HEK293T OTUD4 KD1 cells were transfected with FLAG-TbetaRI C.A. and
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
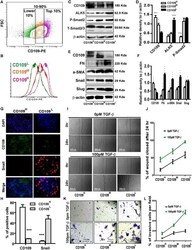
- Figure 2 CD109 expression levels inversely correlate with TGF-beta signaling, EMT marker expression, cellular migration and invasion. ( A ) Isolation of CD109 H , CD109 M , and CD109 L subpopulations of A431 SCC cells by flow cytometry based on their CD109 expression levels. ( B ) Sorted cells were put in culture for three weeks and then re-analyzed by flow cytometry for CD109 expression, which showing that they maintain their respective CD109 expression profiles. ( C ) Representative image and ( D ) quantification of Western blot analysis of TGF-beta receptor I (ALK5) and P-Smad2 in CD109 H , CD109 M CD109 L cells, showing that CD109 expression levels are inversely correlated with TGF-beta signalling. ( E ) Representative image and ( F ) quantification of Western blot analysis for EMT markers in CD109 H , CD109 M , CD109 L cells, respectively. EMT markers expressions are inversely correlated with CD109 expression. ( G ) Representative image and ( H ) quantification of Immunofluorescence microscopy for CD109 (Green), Snail (Red) and DAPI (Blue) in CD109 H and CD109 L SCC cells, respectively, showing that CD109 H cells exhibited decreased Snail expression. ( I ) Representative images and ( J ) quantification of wound-healing assays on CD109 H , CD109 M , and CD109 L subpopulations as indicated, revealing that the levels of CD109 were inversely corelated with the migration of SCC cells. Cell migration was expressed as a percentage of the scratch area filled by migrating cells a
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
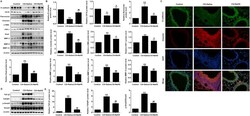
- Fig. 2 NaHS inhibited EMT in CS-exposed mouse lungs. After CS exposure for 12 weeks, mice were euthanized, and lung tissues were collected. (A) Western blot was used to analyze the protein levels of E-cadherin, CK18, Fibronectin, vimentin, FSP-1, alpha-SMA, Snail, MMP-2, MMP-9 and MMP-12 in the lung tissues. (B) Densitometric analysis of E-cadherin, CK18, Fibronectin, vimentin, FSP-1, alpha-SMA, Snail, MMP-2, MMP-9 and MMP-12 in the immunoblots using beta-actin as the internal reference. (C) The expression of E-cadherin and vimentin in the lungs were visualized using immunofluorescence double staining. (D, E) Western blot was used to analyze the protein levels of TGF-beta1, TGFbetaR1 and p-Smad3 in mouse lungs. Data are presented as mean +- SEM of 6 mice/group, *P < 0.05, **P < 0.01, significantly different from control group; # P < 0.05, ## P < 0.01, significantly different from CS + Saline group. Fig. 2
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Other assay
Other assay