Antibody data
- Antibody Data
- Antigen structure
- References [8]
- Comments [0]
- Validations
- Immunocytochemistry [7]
- Immunohistochemistry [4]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-29444 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- TUBA1A Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- Recommended positive controls: 293T, A431, HeLa, HepG2, Jurkat, Raji, HeLa cytosol fraction, Neuro 2A, C8D30, NIH-3T3, Raw264.7, C2C12, PC-12, Rat-2, Drosophila. Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human, Mouse, Rat, Chicken/Avian, Drosophila
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 1.92 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Investigation of niclosamide as a repurposing agent for skeletal muscle atrophy.
The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line.
Factor quinolinone inhibitors alter cell morphology and motility by destabilizing interphase microtubules.
Lithium Chloride Protects against Sepsis-Induced Skeletal Muscle Atrophy and Cancer Cachexia.
Nuclear Import of the HIV-1 Core Precedes Reverse Transcription and Uncoating.
Copy number gains of the putative CRKL oncogene in laryngeal squamous cell carcinoma result in strong nuclear expression of the protein and influence cell proliferation and migration.
Severe biallelic loss-of-function mutations in nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) in two fetuses with fetal akinesia deformation sequence.
Emerin Is Required for Proper Nucleus Reassembly after Mitosis: Implications for New Pathogenetic Mechanisms for Laminopathies Detected in EDMD1 Patients.
Kim HJ, Lee JH, Kim SW, Lee SH, Jung DW, Williams DR
PloS one 2021;16(5):e0252135
PloS one 2021;16(5):e0252135
The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line.
Sakowicz A, Bralewska M, Pietrucha T, Figueras F, Habrowska-Górczyńska DE, Piastowska-Ciesielska AW, Gach A, Sakowicz B, Rybak-Krzyszkowska M, Huras H, Grzesiak M, Biesiada L
International journal of molecular sciences 2021 Sep 22;22(19)
International journal of molecular sciences 2021 Sep 22;22(19)
Factor quinolinone inhibitors alter cell morphology and motility by destabilizing interphase microtubules.
Stoiber P, Scribani Rossi P, Pokharel N, Germany JL, York EA, Schaus SE, Hansen U
Scientific reports 2021 Dec 7;11(1):23564
Scientific reports 2021 Dec 7;11(1):23564
Lithium Chloride Protects against Sepsis-Induced Skeletal Muscle Atrophy and Cancer Cachexia.
Lee JH, Kim SW, Kim JH, Kim HJ, Um J, Jung DW, Williams DR
Cells 2021 Apr 26;10(5)
Cells 2021 Apr 26;10(5)
Nuclear Import of the HIV-1 Core Precedes Reverse Transcription and Uncoating.
Selyutina A, Persaud M, Lee K, KewalRamani V, Diaz-Griffero F
Cell reports 2020 Sep 29;32(13):108201
Cell reports 2020 Sep 29;32(13):108201
Copy number gains of the putative CRKL oncogene in laryngeal squamous cell carcinoma result in strong nuclear expression of the protein and influence cell proliferation and migration.
Kostrzewska-Poczekaj M, Bednarek K, Jarmuz-Szymczak M, Bodnar M, Filas V, Marszalek A, Bartochowska A, Grenman R, Kiwerska K, Szyfter K, Giefing M
Scientific reports 2020 Jan 8;10(1):24
Scientific reports 2020 Jan 8;10(1):24
Severe biallelic loss-of-function mutations in nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) in two fetuses with fetal akinesia deformation sequence.
Lukacs M, Gilley J, Zhu Y, Orsomando G, Angeletti C, Liu J, Yang X, Park J, Hopkin RJ, Coleman MP, Zhai RG, Stottmann RW
Experimental neurology 2019 Oct;320:112961
Experimental neurology 2019 Oct;320:112961
Emerin Is Required for Proper Nucleus Reassembly after Mitosis: Implications for New Pathogenetic Mechanisms for Laminopathies Detected in EDMD1 Patients.
Dubińska-Magiera M, Kozioł K, Machowska M, Piekarowicz K, Filipczak D, Rzepecki R
Cells 2019 Mar 13;8(3)
Cells 2019 Mar 13;8(3)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
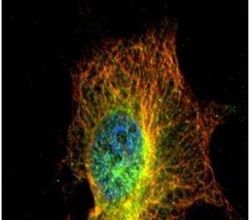
- Experimental details
- Immunofluorescent analysis of alpha Tubulin 4a in methanol-fixed HeLa cells using an Alpha Tubulin 4a polyclonal antibody (Product # PA5-29444) (Green) at a 1:500 dilution. Alpha-tubulin filaments were labeled with Product # PA5-29281 (red) at a 1:2500.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
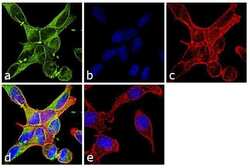
- Experimental details
- Immunofluorescence analysis of alpha Tubulin was performed using 70% confluent log phase NIH/3T3 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Alpha Tubulin Rabbit Polyclonal Antibody (Product # PA5-29544) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
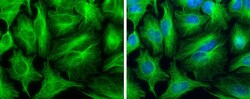
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of alpha Tubulin was performed in HeLa cells fixed in 4% paraformaldehyde at RT for 15 min. Green: alpha Tubulin Polyclonal Antibody (Product # PA5 29444) diluted at 1:500. Blue: Hoechst 33342 staining.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
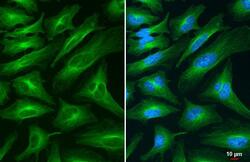
- Experimental details
- alpha Tubulin Polyclonal Antibody detects alpha Tubulin protein at cytoskeleton by immunofluorescent analysis. Sample: HeLa cells were fixed in 4% paraformaldehyde at RT for 15 min. Green: alpha Tubulin stained by alpha Tubulin Polyclonal Antibody (Product # PA5-29444) diluted at 1:500.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
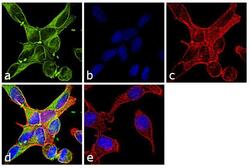
- Experimental details
- Immunofluorescence analysis of alpha Tubulin was performed using 70% confluent log phase NIH/3T3 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Alpha Tubulin Rabbit Polyclonal Antibody (Product # PA5-29544) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
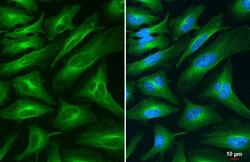
- Experimental details
- alpha Tubulin Polyclonal Antibody detects alpha Tubulin protein at cytoskeleton by immunofluorescent analysis. Sample: HeLa cells were fixed in 4% paraformaldehyde at RT for 15 min. Green: alpha Tubulin stained by alpha Tubulin Polyclonal Antibody (Product # PA5-29444) diluted at 1:500.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
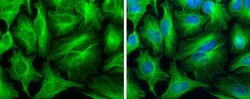
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of alpha Tubulin was performed in HeLa cells fixed in 4% paraformaldehyde at RT for 15 min. Green: alpha Tubulin Polyclonal Antibody (Product # PA5 29444) diluted at 1:500. Blue: Hoechst 33342 staining.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
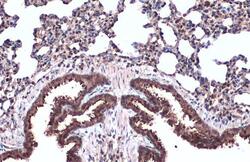
- Experimental details
- TUBA1A Polyclonal Antibody detects alpha Tubulin protein at cell membrane and cytoplasm by immunohistochemical analysis. Sample: Paraffin-embedded mouse lung. alpha Tubulin stained by TUBA1A Polyclonal Antibody (Product # PA5-29444) diluted at 1:500. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
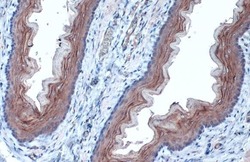
- Experimental details
- TUBA1A Polyclonal Antibody detects alpha Tubulin protein at cell membrane and cytoplasm by immunohistochemical analysis. Sample: Paraffin-embedded rat esophagus. alpha Tubulin stained by TUBA1A Polyclonal Antibody (Product # PA5-29444) diluted at 1:1,000. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
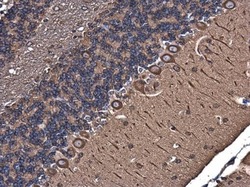
- Experimental details
- Immunohistochemistry (Paraffin) analysis of alpha Tubulin was performed in paraffin-embedded mouse brain tissue using alpha Tubulin Polyclonal Antibody (Product # PA5-29444) at a dilution of 1:500.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
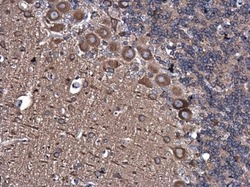
- Experimental details
- Immunohistochemistry (Paraffin) analysis of alpha Tubulin was performed in paraffin-embedded rat brain tissue using alpha Tubulin Polyclonal Antibody (Product # PA5-29444) at a dilution of 1:500.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 5. RT Occurs in the Nuclear Compartment (A and B) A549 (A) and Cf2Th (B) cells were infected with wild-type HIV-1-GFP at an MOI of 2 in the presence of 10 muM nevirapine. After incubation for the indicated times, cells were fractionated, and 10% aliquots of total, cytosolic, and nuclear fractions were analyzed by western blotting using anti-p24, anti-Nopp 140, and anti-alpha-tubulin antibodies (A and B, upper panels) or used for DNA extraction and analyzed for the presence of HIV-1 RT intermediates (early products, minus-strand transfer, intermediate products, and late products) by quantitative PCR as described in STAR Methods (A and B, lower panels). Experiments were repeated three times ( Figures S6 and S7 ), and representative images with standard deviation are shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
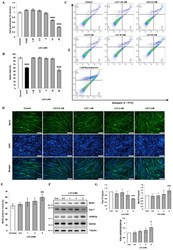
- Figure 1 LiCl enhances myogenic differentiation in C2C12 myoblasts. ( A ) MTT proliferation assay of C2C12 myoblasts after 48 h of treatment with the designated concentration of LiCl (n = 5). ( B ) Quantification results of cell death rates in LiCl-treated C2C12 myoblasts (n = 4). ( C ) Annexin V and PI staining results from C2C12 cells treated with the indicated concentrations of LiCl and staurosphorine. ( D ) Representative immunofluorescence staining images targeting MyHC after 72 h of differentiation following treatment with increasing concentrations of LiCl. Scale bar represents 100 um. ( E ) Measured ratio of MyHC-positive nuclei in immunofluorescence staining (n = 5). ( F ) Representative images of Western blotting for MyHC, Pax-7, phospho (Ser-9) GSK3beta, naive GSK3beta, and alpha-Tubulin after 24 h treatment in differentiation media (DM). ( G ) Quantification results of Western blotting (n = 4). Statistical significance compared to the control is indicated by * (* = p -value < 0.05, ** = p -value < 0.01, *** = p -value < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
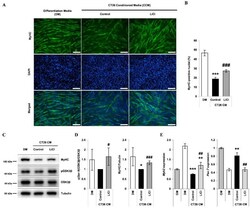
- Figure 2 LiCl ameliorates the inhibition of myoblast differentiation by CT26 colon carcinoma cell-conditioned media (CCM). ( A ) Immunofluorescence staining after 72 h of culture in the following media: (1) differentiation media (DM), (2) CCM only, and (3) CCM and 5 mM LiCl (n = 4). ( B ) Quantified ratio of MyHC-positive nuclei in immunostaining images (n = 4). ( C ) Representative Western blot images for myosin heavy chain (MyHC), phospho (Ser-9) GSK3beta, naive GSK3beta, and alpha-tubulin after 72 h of culture in (1) DM, (2) CCM, (3) CCM and 5 mM LiCl (n = 3). ( D ) Quantification results of Western blot analysis (n = 3). ( E ) qPCR analysis of the myotube marker, Myh2 , and the myoblast marker, Pax-7 , after 24 h of incubation in the following media: (1) GM, (2) DM, (3) CCM, (4) CCM and 5 mM LiCl (n = 5). Statistical significance compared to DM is indicated by * (* = p -value < 0.05, ** = p -value < 0.01, *** = p -value < 0.001). # represents statistical significance compared to the CCM group (# = p -value < 0.05, ## = p -value < 0.01, ### = p -value < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
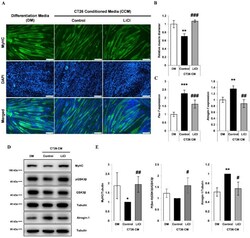
- Figure 3 LiCl prevents CT26 colon carcinoma cell-conditioned media (CCM)-mediated wasting in C2C12 myotubes. ( A ) Immunofluorescence staining for MyHC after 72 h of culture in (1) differentiation media (DM), (2) CCM, and (3) CCM and 5 mM LiCl. DAPI staining was used to visualize cell nuclei. Scale bar = 100 um (n = 4). ( B ) Quantification of relative myotube diameter (n = 4). ( C ) qPCR of Pax-7 and Atrogin-1 expression in myotubes cultured with (1) DM at the start of the experiment, (2) DM for 24 h, (3) CCM for 24 h, and (4) CCM and 5 mM LiCl for 24 h (n = 3). ( D ) Western blotting for MyHC, phospho (Ser-9) GSK3beta, naive GSK3beta, and alpha-Tubulin in C2C12 myotubes after 72 h culture in (1) DM, (2) CCM, and (3) CCM and 5 mM LiCl (n = 4). Western blotting for Atrogin-1 and alpha-Tubulin (lower) in C2C12 myotubes after 24 h culture in (1) DM, (2) CCM, and (3) CCM and 5 mM LiCl (n = 3). ( E ) Quantification results of Western blotting for MyHC (n = 4), phospho (Ser-9) GSK3beta (n = 3), and Atrogin-1 (n = 3). Significance compared to DM treatment is indicated by * (* = p -value < 0.05, ** = p -value < 0.01, *** = p -value < 0.001). Significance compared to CCM treatment is indicated by # (# = p -value < 0.05) ## = p -valve < 0.01, ### = p -value < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
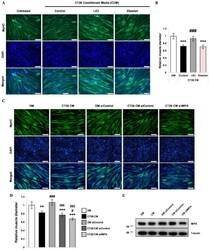
- Figure 4 Effect of the lithium mimetic and IMPase inhibitor, ebselen, on CCM-induced wasting in C2C12 myotubes ( A ) Immunofluorescence staining for MyHC after 72 h of culture in (1) DM, (2) CCM, (3) CCM and 5 mM LiCl, and (4) CCM and 10 uM ebselen. DAPI staining was used to visualize cell nuclei. Scale bar = 100 um. (n = 3). ( B ) Quantification of relative myotube diameter. (n = 3). ( C ) Representative immunofluorescence staining images for MyHC after 72 h of the designated treatments. ( D ) Quantification of relative myotube diameters (n = 4). ( E ) Representative Western blot images for IMPA (n = 4). Significance compared to DM is indicated by * (** = p -value < 0.01, *** = p -value < 0.001). Significance compared to CT26 CM (CCM) treatment is indicated by # (# = p -value < 0.05, ### = p -value < 0.001). Statistical significance relative to siControl-treated DM is indicated by $ ($$$ = p -value < 0.001).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
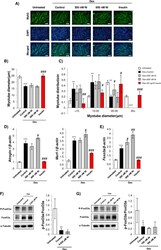
- 10.1371/journal.pone.0252135.g002 Fig 2 A) Representative images of myosin heavy chain immunostained C2C12 myotubes after treatment with Dex and Ni (scale bar = 100 mum). Myoblasts were differentiated for 72 h and treated with 10 muM Dex, or 10 muM Dex plus 300 nM or 600 nM Ni or 50 ng/ml bovine insulin for 24 h. B) Average diameter of C2C12 myotubes treated with Dex or Dex+Ni. *** = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
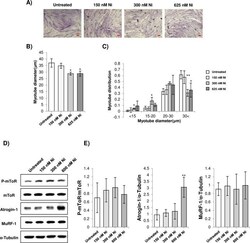
- 10.1371/journal.pone.0252135.g003 Fig 3 A) Representative images of H&E stained C2C12 myotubes treated with Ni (scale bar = 100 mum). Myoblasts were differentiated for 72 h and treated with 150 nM, 300 nM, and 625 nM Ni for 24 h. B-C) Average diameter and myotube diameter distribution of C2C12 myotubes treated with Ni. * = p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
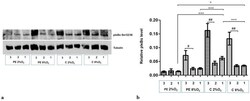
- Figure 5 Western blot results for pIkappaBalpha Ser32/36 in the cytoplasmic fraction after MG-132 stimulation. The cells were cultured for 96 h in hypoxic or normoxic conditions with medium containing 1% serum from preeclamptic or normotensive women. For the last three hours, the culture medium was supplemented with the following factors: MG-132 (3), DMSO as the MG-132 vehicle (2) or none, i.e., cells incubated only with medium containing maternal serum without MG-132 or DMSO addition (1). ( a ) The Western blot membrane with pIkappaBalpha and Tubulin as a loading control ( b ) the analysis of Western blot results. Values are presented as mean +- SEM; ( n = 3 experiments). The differences in pIkappaBalpha level between cells with DMSO and MG-132 were analyzed by Student's t -test, # p < 0.05, ## p < 0.01. The difference in pIkBalpha level for cells not stimulated by DMSO or MG-132 but grown in hypoxia/normoxia and control/preeclamptic serum were analysed by ANOVA with the Bonferroni post hoc test; * p < 0.05, *** p < 0.001.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry