14-3219-95
antibody from Invitrogen Antibodies
Targeting: F11R
CD321, JAM-1, JAM-A, JAM1, JAMA, JCAM, PAM-1
Antibody data
- Antibody Data
- Antigen structure
- References [2]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [2]
- Flow cytometry [1]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-3219-95 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD321 (F11R) Monoclonal Antibody (CSTEM27), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- This monoclonal antibody CSTEM27 reacts with human CD321 (F11R, JAM-1, JAM-A).
- Antibody clone number
- CSTEM27
- Concentration
- 0.5 mg/mL
Submitted references SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins That Maintain Endothelial Barrier Integrity.
Retinal Distribution and Extracellular Activity of Granzyme B: A Serine Protease That Degrades Retinal Pigment Epithelial Tight Junctions and Extracellular Matrix Proteins.
Raghavan S, Kenchappa DB, Leo MD
Frontiers in cardiovascular medicine 2021;8:687783
Frontiers in cardiovascular medicine 2021;8:687783
Retinal Distribution and Extracellular Activity of Granzyme B: A Serine Protease That Degrades Retinal Pigment Epithelial Tight Junctions and Extracellular Matrix Proteins.
Matsubara JA, Tian Y, Cui JZ, Zeglinski MR, Hiroyasu S, Turner CT, Granville DJ
Frontiers in immunology 2020;11:574
Frontiers in immunology 2020;11:574
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
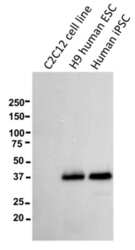
- Experimental details
- Western blot of reduced murine C2C12 myoblast cells, H9 human ESCs, or human iPSCs using 5 µg/mL of Anti-Human CD321 (F11R) Purified antibody. Bands were visualized using Anti-Mouse IgG HRP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
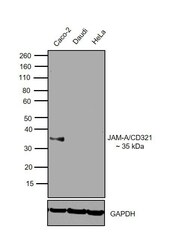
- Experimental details
- Western blot was performed using Anti-CD321 Mouse Monoclonal Antibody (Product # 14-32-1982) and 35 kDa band corresponding to CD321 was observed across cell lines tested except Daudi and HeLa. Membrane enriched extracts (30 µg lysate) of Caco-2 (Lane 1), Daudi (Lane 2) and HeLa (Lane 3) were electrophoresed using NuPAGE™ 4-12% Bis-Tris Protein Gel (Product # NP0322BOX). Resolved proteins were then transferred onto a nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (5 µg/mL) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, HRP (Product # A28177, 1:4000 dilution) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
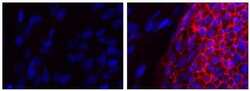
- Experimental details
- Immunocytochemistry of fixed and permeabilized human iPSCs plated on murine derived feeder cells using 5 µg/mL Mouse IgG2a K Isotype Control Purified (Product # 14-4724-82) (left) or 5 µg/mL Anti-CD321 (F11R) Purified (right) followed by F (ab’)2-Goat anti-Mouse IgG Secondary Antibody eFluor® 660 (Product # 50-4010-82). Nuclei are stained with DAPI. An iPSC colony (positive staining) and murine derived feeder cells (negative staining) are in both fields of view above.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
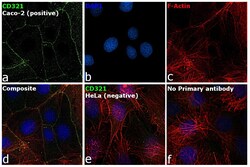
- Experimental details
- Immunofluorescence analysis of CD321 was performed using 70% confluent log phase Caco-2 and HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with CD321 Monoclonal Antibody (Product # 14-3219-82) at 5 µg/mL in 0.1% BSA and incubated overnight at 4 degree and then labeled with Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green) in Caco-2 cells. Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image of Caco-2 cells, which is a positive model for CD321 expression showing a membrane junction localization. Panel e represents the merged image of HeLa cells, that are null for CD321 protein expression. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
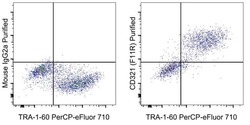
- Experimental details
- Staining of a mixture of human iPSC and the C2C12 murine cell line with Anti-Human TRA-1-60 PerCP-eFluor 710 (Product # 46-8863-82) and 0.25 µg Mouse IgG2a K Isotype Control Purified (Product # 14-4724-82) (left) or 0.25 µg of Anti-Human CD321 (F11R) Purified (right) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4010-82).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
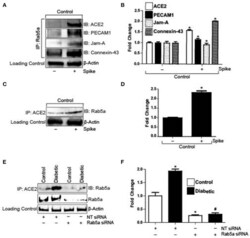
- Experimental details
- Figure 2 Spike increases Rab5a association with ACE2 and junctional proteins. (A) Representative Western blot of coimmunoprecipitation experiments done using Rab5a antibody and probed for different proteins. (B) Mean data indicating fold change in Rab5a association with junctional proteins and ACE2 after S1RBD treatment. n = 6 for each, * P < 0.05 vs. untreated control. (C) Representative Western blot of coimmunoprecipitation experiments done using ACE2 antibody and probed for Rab5a. (D) Mean data indicating fold change in ACE2 association with Rab5a after Spike treatment. n = 6 for each, * P < 0.05 vs. untreated control. (E) Representative Western blot of coimmunoprecipitation experiments in control and diabetic cells after Rab5a knockdown with siRNA. (F) Mean data indicating fold change in ACE2 association with Rab5a after Rab5a siRNA. n = 6 for each, * P < 0.05 vs. control, # P < 0.05 vs. NT siRNA diabetic.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
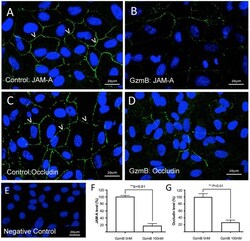
- Experimental details
- Figure 6 Reduced immunoreactivity of JAM-A and Occludin in ARPE-19 cells stimulated by exogenous GzmB. (A) JAM-A immunoreactivity demonstrates tight junctional contacts (green 488 nm, some shown by white arrowheads) on RPE in controls wells (GzmB = 0 nM). (B) After 6 h with GzmB stimulation (100 nM), JAM-A immunoreactivity is reduced on ARPE-19. (C) Occludin immunoreactivity between cells (some shown by white arrowheads) in control wells (GzmB = 0 nM). (D) After 6 h with GzmB stimulation (100 nM), Occludin immunoreactivity is reduced on ARPE-19. (E) Omission of primary antibody demonstrates no immunoreactivity. Significant differences were observed for (F) JAM-A and (G) Occludin using an independent-sample T -Test ( N = 6 per group) between control and GzmB-treated group: mean +- SEM; ** p < 0.01. Images were taken from ARPE-19 cells and are representative of primary RPE experiments undertaken at the same time. DAPI (405 nm) labeling of nuclei is shown in blue. Scale bar = 20 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot