Antibody data
- Antibody Data
- Antigen structure
- References [21]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 44-422G - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-JAK1 (Tyr1034, Tyr1035) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This product was formerly called "Phospho-JAK1 (Tyr1022, Tyr1023) Polyclonal Antibody" because Human Jak1 residues Tyr1034 and Tyr1035 have been historically referenced as Tyr1022 and Tyr1023.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Storage
- -20°C
Submitted references Drug target validation in primary human natural killer cells using CRISPR RNP.
Sulfated non-anticoagulant heparin blocks Th2-induced asthma by modulating the IL-4/signal transducer and activator of transcription 6/Janus kinase 1 pathway.
ATM‑JAK‑PD‑L1 signaling pathway inhibition decreases EMT and metastasis of androgen‑independent prostate cancer.
ALK-activating homologous mutations in LTK induce cellular transformation.
IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway.
IL-2 induces conformational changes in its preassembled receptor core, which then migrates in lipid raft and binds to the cytoskeleton meshwork.
Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response.
Transforming JAK1 mutations exhibit differential signalling, FERM domain requirements and growth responses to interferon-γ.
SOCS-1 binding to tyrosine 441 of IFN-gamma receptor subunit 1 contributes to the attenuation of IFN-gamma signaling in vivo.
'Tuning' of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages.
Molecular mechanisms involved in interleukin-4-induced human neutrophils: expression and regulation of suppressor of cytokine signaling.
Erythropoietin protects the infant heart against ischemia-reperfusion injury by triggering multiple signaling pathways.
Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes.
Role of tyrosine 441 of interferon-gamma receptor subunit 1 in SOCS-1-mediated attenuation of STAT1 activation.
Mumps virus V protein antagonizes interferon without the complete degradation of STAT1.
Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin.
Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1.
Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1.
Functional expression of the interleukin-11 receptor alpha-chain and evidence of antiapoptotic effects in human colonic epithelial cells.
C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein.
C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein.
Rautela J, Surgenor E, Huntington ND
Journal of leukocyte biology 2020 Oct;108(4):1397-1408
Journal of leukocyte biology 2020 Oct;108(4):1397-1408
Sulfated non-anticoagulant heparin blocks Th2-induced asthma by modulating the IL-4/signal transducer and activator of transcription 6/Janus kinase 1 pathway.
Ghonim MA, Wang J, Ibba SV, Luu HH, Pyakurel K, Benslimane I, Mousa S, Boulares AH
Journal of translational medicine 2018 Sep 1;16(1):243
Journal of translational medicine 2018 Sep 1;16(1):243
ATM‑JAK‑PD‑L1 signaling pathway inhibition decreases EMT and metastasis of androgen‑independent prostate cancer.
Zhang L, Xu LJ, Zhu J, Li J, Xue BX, Gao J, Sun CY, Zang YC, Zhou YB, Yang DR, Shan YX
Molecular medicine reports 2018 May;17(5):7045-7054
Molecular medicine reports 2018 May;17(5):7045-7054
ALK-activating homologous mutations in LTK induce cellular transformation.
Roll JD, Reuther GW
PloS one 2012;7(2):e31733
PloS one 2012;7(2):e31733
IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway.
Yadav A, Kumar B, Datta J, Teknos TN, Kumar P
Molecular cancer research : MCR 2011 Dec;9(12):1658-67
Molecular cancer research : MCR 2011 Dec;9(12):1658-67
IL-2 induces conformational changes in its preassembled receptor core, which then migrates in lipid raft and binds to the cytoskeleton meshwork.
Pillet AH, Lavergne V, Pasquier V, Gesbert F, Thèze J, Rose T
Journal of molecular biology 2010 Nov 12;403(5):671-92
Journal of molecular biology 2010 Nov 12;403(5):671-92
Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response.
Rose T, Pillet AH, Lavergne V, Tamarit B, Lenormand P, Rousselle JC, Namane A, Thèze J
The Journal of biological chemistry 2010 May 14;285(20):14898-908
The Journal of biological chemistry 2010 May 14;285(20):14898-908
Transforming JAK1 mutations exhibit differential signalling, FERM domain requirements and growth responses to interferon-γ.
Gordon GM, Lambert QT, Daniel KG, Reuther GW
The Biochemical journal 2010 Dec 1;432(2):255-65
The Biochemical journal 2010 Dec 1;432(2):255-65
SOCS-1 binding to tyrosine 441 of IFN-gamma receptor subunit 1 contributes to the attenuation of IFN-gamma signaling in vivo.
Starr R, Fuchsberger M, Lau LS, Uldrich AP, Goradia A, Willson TA, Verhagen AM, Alexander WS, Smyth MJ
Journal of immunology (Baltimore, Md. : 1950) 2009 Oct 1;183(7):4537-44
Journal of immunology (Baltimore, Md. : 1950) 2009 Oct 1;183(7):4537-44
'Tuning' of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages.
Wang L, Tassiulas I, Park-Min KH, Reid AC, Gil-Henn H, Schlessinger J, Baron R, Zhang JJ, Ivashkiv LB
Nature immunology 2008 Feb;9(2):186-93
Nature immunology 2008 Feb;9(2):186-93
Molecular mechanisms involved in interleukin-4-induced human neutrophils: expression and regulation of suppressor of cytokine signaling.
Ratthé C, Pelletier M, Chiasson S, Girard D
Journal of leukocyte biology 2007 May;81(5):1287-96
Journal of leukocyte biology 2007 May;81(5):1287-96
Erythropoietin protects the infant heart against ischemia-reperfusion injury by triggering multiple signaling pathways.
Rafiee P, Shi Y, Su J, Pritchard KA Jr, Tweddell JS, Baker JE
Basic research in cardiology 2005 May;100(3):187-97
Basic research in cardiology 2005 May;100(3):187-97
Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes.
Shiao YJ, Lin YL, Sun YH, Chi CW, Chen CF, Wang CN
British journal of pharmacology 2005 Jan;144(1):42-51
British journal of pharmacology 2005 Jan;144(1):42-51
Role of tyrosine 441 of interferon-gamma receptor subunit 1 in SOCS-1-mediated attenuation of STAT1 activation.
Qing Y, Costa-Pereira AP, Watling D, Stark GR
The Journal of biological chemistry 2005 Jan 21;280(3):1849-53
The Journal of biological chemistry 2005 Jan 21;280(3):1849-53
Mumps virus V protein antagonizes interferon without the complete degradation of STAT1.
Kubota T, Yokosawa N, Yokota S, Fujii N, Tashiro M, Kato A
Journal of virology 2005 Apr;79(7):4451-9
Journal of virology 2005 Apr;79(7):4451-9
Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin.
Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D, Marrero MB
American journal of physiology. Cell physiology 2005 Apr;288(4):C805-12
American journal of physiology. Cell physiology 2005 Apr;288(4):C805-12
Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1.
Yokota S, Okabayashi T, Yokosawa N, Fujii N
Journal of virology 2004 May;78(9):4591-8
Journal of virology 2004 May;78(9):4591-8
Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1.
Yokota S, Okabayashi T, Yokosawa N, Fujii N
Journal of virology 2004 May;78(9):4591-8
Journal of virology 2004 May;78(9):4591-8
Functional expression of the interleukin-11 receptor alpha-chain and evidence of antiapoptotic effects in human colonic epithelial cells.
Kiessling S, Muller-Newen G, Leeb SN, Hausmann M, Rath HC, Strater J, Spottl T, Schlottmann K, Grossmann J, Montero-Julian FA, Scholmerich J, Andus T, Buschauer A, Heinrich PC, Rogler G
The Journal of biological chemistry 2004 Mar 12;279(11):10304-15
The Journal of biological chemistry 2004 Mar 12;279(11):10304-15
C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein.
Yokosawa N, Yokota S, Kubota T, Fujii N
Journal of virology 2002 Dec;76(24):12683-90
Journal of virology 2002 Dec;76(24):12683-90
C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein.
Yokosawa N, Yokota S, Kubota T, Fujii N
Journal of virology 2002 Dec;76(24):12683-90
Journal of virology 2002 Dec;76(24):12683-90
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
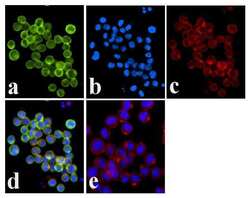
- Experimental details
- Immunofluorescence analysis of JAK1 (pY1022/1023) was done on 70% confluent log phase COLO 205 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with JAK1 (pY1022/1023) Rabbit polyclonal Antibody (Product # 44-422G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing membrane localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
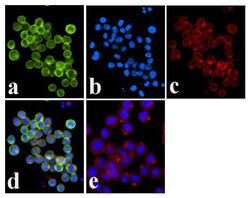
- Experimental details
- Immunofluorescence analysis of JAK1 (pY1022/1023) was done on 70% confluent log phase COLO 205 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with JAK1 (pY1022/1023) Rabbit polyclonal Antibody (Product # 44-422G) at 1:250 dilution in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Goat Anti-Rabbit IgG Secondary Antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing membrane localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
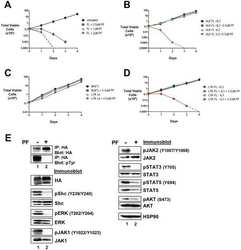
- Experimental details
- Figure 6 Transformed LTK-F568L BaF3 cells are sensitive to the ALK inhibitor PF-2341066. ( A ) BaF3 cells transformed to cytokine independence by LTK-F568L were left untreated (0.1% DMSO, black circles) or cultured in the presence of 0.5 uM (blue squares), 1 uM (green triangles), or 2 uM (red inverted triangles) of the cMET/ALK inhibitor PF2341066 (PF) on Day 0. The total number of viable cells for each treatment was determined daily by trypan blue exclusion. ( B ) IL-3-dependent (+IL-3) BaF3 cells harboring ALK-F1174L were cultured without (black circles) or with (blue squares) 0.5 uM PF2341066. BaF3 cells transformed to IL-3-independence by ALK-F1174L were cultured without (green triangles) or with (red inverted triangles) 0.5 uM PF2341066. The total number of viable cells for each treatment was determined daily by trypan blue exclusion. ( C ) Parental BaF3 cells were cultured without (black circles) or with (blue squares) 0.5 uM PF2341066. BaF3 cells expressing wildtype LTK were cultured without (green triangles) or with (red inverted triangles) 0.5 uM PF2341066. The total number of viable cells for each treatment was determined daily by trypan blue exclusion. ( D ) IL-3-dependent BaF3 cells expressing LTK-F568L were cultured without (black inverted triangles) or with (blue circles) 0.5 uM PF2341066. BaF3 cells transformed to IL-3 independence by LTK-F568L were cultured without (green squares) or with (red diamonds) 0.5 uM PF2341066. The total number of viable cells for ea
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
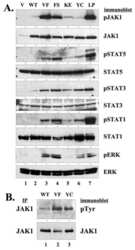
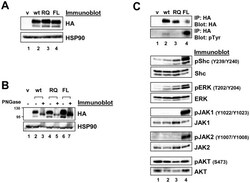
- Figure 2 Expression of LTK constructs in 293T cells. ( A ) Relative protein expression of wildtype and mutant LTK constructs in 293T cells. Lysates of 293T cells transiently expressing either HA-tagged vector control (v, lane 1), wildtype LTK (wt, lane 2), LTK-R669Q (RQ, lane 3), or LTK-F568L (FL, lane 4) were immunoblotted with an anti-HA antibody as well as HSP90 as a loading control. ( B ) 293T cell lysates containing the LTK proteins were treated with PNGase F (lanes 3, 5, and 7) or left untreated (lanes 2, 4, and 6) and immunoblotted with HA antibodies and HSP90 as a loading control. ( C ) HA-tagged LTK proteins expressed in 293T cells were immunoprecipitated and immunoblotted with HA antibodies as well as anti-phosphotyrosine (pTyr) antibodies (top). Antibodies that recognize the indicated proteins (Shc, pShC-Y239/Y240, ERK, pERK-T202/Y204, JAK1, pJAK1-Y1022/Y1023, JAK2, pJAK2-Y1007/Y1008, AKT, and pAKT-S473) were used to immunoblot cell lysates of 293T cells expressing LTK proteins (bottom). The phosphorylated form of the protein is designated with a ""p"" preceding the protein name.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
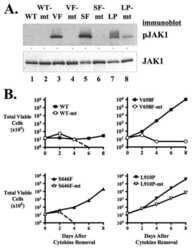
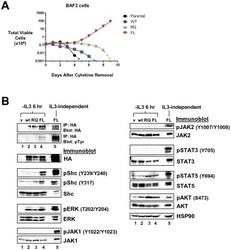
- Figure 3 LTK-F568L transforms BaF3 cells to cytokine-independence. ( A ) BaF3 cells stably expressing LTK-F568L (red inverted triangles), LTK-R669Q (green triangles), or wildtype LTK (blue squares) and parental control cells (black circles) were cultured in the absence of IL-3 at Day 0. The total number of viable cells was determined at each timepoint by trypan blue exclusion. Similar results were obtained in three independent experiments. The dashed line indicates cell numbers going below the detection of the hemacytometer towards zero. ( B ) Lysates from cells stably expressing either HA-tagged vector control (v, lane 1), wildtype LTK (wt, lane 2), LTK-R669Q (RQ, lane 3), or LTK-F568L (FL, lane 4) were cultured in the absence of IL-3 for six hours and immunoprecipitated with HA antibodies, followed by immunoblotting with HA and pTyr antibodies (first two blots). The same lysates were immunoblotted with antibodies to the indicated cell signaling proteins (see Figure 2 legend plus pShc-Y317, STAT3, pSTAT3-Y705, STAT5 and pSTAT5-Y694). Cell lysate from IL-3-independent LTK-F568L-expressing BaF3 cells was also analyzed (lane 5).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
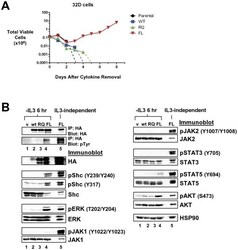
- Figure 4 LTK-F568L transforms 32D cells to cytokine-independence. ( A ) 32D cells stably expressing LTK-F568L (red inverted triangles), LTK-R669Q (green triangles), or wildtype LTK (blue squares) and parental control cells (black circles) were cultured in the absence of IL-3 at Day 0. The total number of viable cells was determined at each timepoint by trypan blue exclusion. Similar results were obtained in three independent experiments. The dashed line indicates cell numbers going below the detection of the hemacytometer towards zero. ( B ) Lysates from cells stably expressing either HA-tagged vector control (v, lane 1), wildtype LTK (wt, lane 2), LTK-R669Q (RQ, lane 3), or LTK-F568L (FL, lane 4) were cultured in the absence of IL-3 for six hours and immunoprecipitated with HA antibodies, followed by immunoblotting with HA and pTyr antibodies (first two blots). The same lysates were immunoblotted with antibodies to the indicated cell signaling proteins (See Figure 3 legend). Cell lysate from IL-3-independent LTK-F568L-expressing 32D cells was also analyzed (lane 5).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
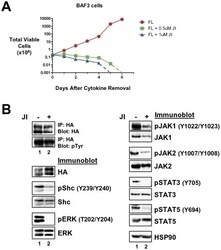
- Figure 5 Transformation of BaF3 cells by LTK-F568L requires JAK family kinase activity. ( A ) BaF3 cells transformed to cytokine independence by LTK-F568L were cultured in the presence of 0.5 uM (green squares) or 1 uM (blue triangles) JAK Inhibitor I (JI) or 0.1% DMSO (red circles) on Day 0. The total number of viable cells was determined by trypan blue exclusion. Dashed lines indicate viable cell counts going below the limit of detection of the hemacytometer towards zero. Similar results were obtained in two independent experiments. ( B ) IL-3-independent LTK-F568L BaF3 cells were treated with 1 uM (lane 2) JI or DMSO (lane 1) for three hours and immunoblotted for the indicated proteins (See Figure 3 legend). Phosphotyrosine (pTyr) on LTK-F568L was detected by immunoblotting following immunoprecipitating LTK-F568L with HA antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
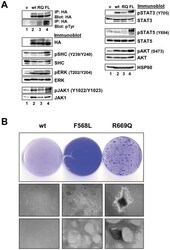
- Figure 7 LTK-F568L and LTK-R669Q transform epithelial cells. ( A ) Cell lysates from RIE cells stably expressing either empty vector (v, lane 1), wildtype LTK (wt, lane 2), LTK-R669Q (RQ, lane 3), or LTK-F568L (FL, lane 4) were analyzed by immunoprecipitation and immunoblotting, as indicated (see Figure 3 legend). ( B ) RIE cells stably expressing either wildtype LTK (wt), LTK-F568L (F568L), or LTK-R669Q (R669Q) were plated and reached confluency after six days. Cells were cultured further and growth was grossly assessed for the loss of contact inhibition. Cells expressing wildtype LTK did not grow beyond confluency, continuing to exhibit contact inhibition. However, cells expressing LTK-F568L continued to proliferate, forming swirling patterns of cells growing on top of the monolayer. Cells expressing LTK-R669Q formed distinct secondary morphological structures as these cells formed compact clusters of cells that grew on top of the monolayer of cells. Representative photographs of cells are shown: plates were stained with crystal violet (top row) after photographs were taken at 10x with a digital camera (middle) and 50x with a Zeiss Automated Fluorescent Microscope using AxioVision (bottom row). Results shown are representative of similar findings of three independent experiments.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry