Antibody data
- Antibody Data
- Antigen structure
- References [22]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Immunoprecipitation [1]
- Immunohistochemistry [5]
- Flow cytometry [6]
- Chromatin Immunoprecipitation [2]
- Other assay [10]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-014 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- SOX2 Monoclonal Antibody (20G5)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- Western blot analysis of MA1-014 specifically detects SOX2 protein at ~36 kDa in the nucleus of human and mouse embryonal carcinoma and iPS cells.
- Reactivity
- Human, Mouse, Canine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 20G5
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C
Submitted references Exopolysaccharide ID1 Improves Post-Warming Outcomes after Vitrification of In Vitro-Produced Bovine Embryos.
Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response.
Glycoproteomic analysis of the changes in protein N-glycosylation during neuronal differentiation in human-induced pluripotent stem cells and derived neuronal cells.
Novel fragile X syndrome 2D and 3D brain models based on human isogenic FMRP-KO iPSCs.
Deconvolution of cell type-specific drug responses in human tumor tissue with single-cell RNA-seq.
Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation.
Expression of ALDH and SOX-2 in Pulmonary Sclerosing Pnemocytoma (PSP) of the Lung: Is There a Meaning Behind?
Cancer Stem-Like Cells in a Case of an Inflammatory Myofibroblastic Tumor of the Lung.
Generation of Complete Multi-Cell Type Lung Organoids From Human Embryonic and Patient-Specific Induced Pluripotent Stem Cells for Infectious Disease Modeling and Therapeutics Validation.
Neddylation is critical to cortical development by regulating Wnt/β-catenin signaling.
EGF and a GSK3 Inhibitor Deplete Junctional E-cadherin and Stimulate Proliferation in the Mature Mammalian Ear.
Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche.
Dysfunction of iPSC-derived endothelial cells in human Hutchinson-Gilford progeria syndrome.
Cancer stem-neuroendocrine cells in an atypical carcinoid case report.
GPD1 Specifically Marks Dormant Glioma Stem Cells with a Distinct Metabolic Profile.
MELK inhibition targets cancer stem cells through downregulation of SOX2 expression in head and neck cancer cells.
Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes.
Generation of two human isogenic iPSC lines from fetal dermal fibroblasts.
Disentangling the aneuploidy and senescence paradoxes: a study of triploid breast cancers non-responsive to neoadjuvant therapy.
Conservative site-specific and single-copy transgenesis in human LINE-1 elements.
Role of stress-activated OCT4A in the cell fate decisions of embryonal carcinoma cells treated with etoposide.
Hopx distinguishes hippocampal from lateral ventricle neural stem cells.
Ordóñez-León EA, Martínez-Rodero I, García-Martínez T, López-Béjar M, Yeste M, Mercade E, Mogas T
International journal of molecular sciences 2022 Jun 25;23(13)
International journal of molecular sciences 2022 Jun 25;23(13)
Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response.
Warrier NM, Krishnan RK, Prabhu V, Hariharapura RC, Agarwal P, Kumar P
International journal of molecular sciences 2022 Jul 9;23(14)
International journal of molecular sciences 2022 Jul 9;23(14)
Glycoproteomic analysis of the changes in protein N-glycosylation during neuronal differentiation in human-induced pluripotent stem cells and derived neuronal cells.
Kimura K, Koizumi T, Urasawa T, Ohta Y, Takakura D, Kawasaki N
Scientific reports 2021 May 27;11(1):11169
Scientific reports 2021 May 27;11(1):11169
Novel fragile X syndrome 2D and 3D brain models based on human isogenic FMRP-KO iPSCs.
Brighi C, Salaris F, Soloperto A, Cordella F, Ghirga S, de Turris V, Rosito M, Porceddu PF, D'Antoni C, Reggiani A, Rosa A, Di Angelantonio S
Cell death & disease 2021 May 15;12(5):498
Cell death & disease 2021 May 15;12(5):498
Deconvolution of cell type-specific drug responses in human tumor tissue with single-cell RNA-seq.
Zhao W, Dovas A, Spinazzi EF, Levitin HM, Banu MA, Upadhyayula P, Sudhakar T, Marie T, Otten ML, Sisti MB, Bruce JN, Canoll P, Sims PA
Genome medicine 2021 May 11;13(1):82
Genome medicine 2021 May 11;13(1):82
Guided Self-Assembly of ES-Derived Lung Progenitors into Biomimetic Tube Structures That Impact Cell Differentiation.
Soleas JP, Huang L, D'Arcangelo E, Nostro MC, Waddell TK, McGuigan AP, Karoubi G
Bioengineering (Basel, Switzerland) 2021 Dec 10;8(12)
Bioengineering (Basel, Switzerland) 2021 Dec 10;8(12)
Expression of ALDH and SOX-2 in Pulmonary Sclerosing Pnemocytoma (PSP) of the Lung: Is There a Meaning Behind?
Aramini B, Masciale V, Manfredini B, Bianchi D, Banchelli F, D'Amico R, Bertolini F, Dominici M, Morandi U, Maiorana A
Frontiers in medicine 2020;7:497
Frontiers in medicine 2020;7:497
Cancer Stem-Like Cells in a Case of an Inflammatory Myofibroblastic Tumor of the Lung.
Masciale V, Grisendi G, Banchelli F, D'Amico R, Maiorana A, Sighinolfi P, Brugioni L, Stefani A, Morandi U, Dominici M, Aramini B
Frontiers in oncology 2020;10:673
Frontiers in oncology 2020;10:673
Generation of Complete Multi-Cell Type Lung Organoids From Human Embryonic and Patient-Specific Induced Pluripotent Stem Cells for Infectious Disease Modeling and Therapeutics Validation.
Leibel SL, McVicar RN, Winquist AM, Niles WD, Snyder EY
Current protocols in stem cell biology 2020 Sep;54(1):e118
Current protocols in stem cell biology 2020 Sep;54(1):e118
Neddylation is critical to cortical development by regulating Wnt/β-catenin signaling.
Zhang L, Jing H, Li H, Chen W, Luo B, Zhang H, Dong Z, Li L, Su H, Xiong WC, Mei L
Proceedings of the National Academy of Sciences of the United States of America 2020 Oct 20;117(42):26448-26459
Proceedings of the National Academy of Sciences of the United States of America 2020 Oct 20;117(42):26448-26459
EGF and a GSK3 Inhibitor Deplete Junctional E-cadherin and Stimulate Proliferation in the Mature Mammalian Ear.
Kozlowski MM, Rudolf MA, Corwin JT
The Journal of neuroscience : the official journal of the Society for Neuroscience 2020 Mar 25;40(13):2618-2632
The Journal of neuroscience : the official journal of the Society for Neuroscience 2020 Mar 25;40(13):2618-2632
Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche.
Bassi G, Panseri S, Dozio SM, Sandri M, Campodoni E, Dapporto M, Sprio S, Tampieri A, Montesi M
Scientific reports 2020 Dec 18;10(1):22294
Scientific reports 2020 Dec 18;10(1):22294
Dysfunction of iPSC-derived endothelial cells in human Hutchinson-Gilford progeria syndrome.
Matrone G, Thandavarayan RA, Walther BK, Meng S, Mojiri A, Cooke JP
Cell cycle (Georgetown, Tex.) 2019 Oct;18(19):2495-2508
Cell cycle (Georgetown, Tex.) 2019 Oct;18(19):2495-2508
Cancer stem-neuroendocrine cells in an atypical carcinoid case report.
Masciale V, Grisendi G, Banchelli F, D'Amico R, Maiorana A, Morandi U, Dominici M, Aramini B
Translational lung cancer research 2019 Dec;8(6):1157-1162
Translational lung cancer research 2019 Dec;8(6):1157-1162
GPD1 Specifically Marks Dormant Glioma Stem Cells with a Distinct Metabolic Profile.
Rusu P, Shao C, Neuerburg A, Acikgöz AA, Wu Y, Zou P, Phapale P, Shankar TS, Döring K, Dettling S, Körkel-Qu H, Bekki G, Costa B, Guo T, Friesen O, Schlotter M, Heikenwalder M, Tschaharganeh DF, Bukau B, Kramer G, Angel P, Herold-Mende C, Radlwimmer B, Liu HK
Cell stem cell 2019 Aug 1;25(2):241-257.e8
Cell stem cell 2019 Aug 1;25(2):241-257.e8
MELK inhibition targets cancer stem cells through downregulation of SOX2 expression in head and neck cancer cells.
Ren L, Deng B, Saloura V, Park JH, Nakamura Y
Oncology reports 2019 Apr;41(4):2540-2548
Oncology reports 2019 Apr;41(4):2540-2548
Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes.
El-Battrawy I, Lan H, Cyganek L, Zhao Z, Li X, Buljubasic F, Lang S, Yücel G, Sattler K, Zimmermann WH, Utikal J, Wieland T, Ravens U, Borggrefe M, Zhou XB, Akin I
Journal of the American Heart Association 2018 Mar 24;7(7)
Journal of the American Heart Association 2018 Mar 24;7(7)
Generation of two human isogenic iPSC lines from fetal dermal fibroblasts.
Tandon R, Brändl B, Baryshnikova N, Landshammer A, Steenpaß L, Keminer O, Pless O, Müller FJ
Stem cell research 2018 Dec;33:120-124
Stem cell research 2018 Dec;33:120-124
Disentangling the aneuploidy and senescence paradoxes: a study of triploid breast cancers non-responsive to neoadjuvant therapy.
Gerashchenko BI, Salmina K, Eglitis J, Huna A, Grjunberga V, Erenpreisa J
Histochemistry and cell biology 2016 Apr;145(4):497-508
Histochemistry and cell biology 2016 Apr;145(4):497-508
Conservative site-specific and single-copy transgenesis in human LINE-1 elements.
Vijaya Chandra SH, Makhija H, Peter S, Myint Wai CM, Li J, Zhu J, Ren Z, D'Alcontres MS, Siau JW, Chee S, Ghadessy FJ, Dröge P
Nucleic acids research 2016 Apr 7;44(6):e55
Nucleic acids research 2016 Apr 7;44(6):e55
Role of stress-activated OCT4A in the cell fate decisions of embryonal carcinoma cells treated with etoposide.
Huna A, Salmina K, Erenpreisa J, Vazquez-Martin A, Krigerts J, Inashkina I, Gerashchenko BI, Townsend PA, Cragg MS, Jackson TR
Cell cycle (Georgetown, Tex.) 2015;14(18):2969-84
Cell cycle (Georgetown, Tex.) 2015;14(18):2969-84
Hopx distinguishes hippocampal from lateral ventricle neural stem cells.
Li D, Takeda N, Jain R, Manderfield LJ, Liu F, Li L, Anderson SA, Epstein JA
Stem cell research 2015 Nov;15(3):522-529
Stem cell research 2015 Nov;15(3):522-529
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
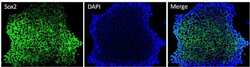
- Experimental details
- Immunofluorescent analysis of Sox2 (green) in HEL 11.4 induced IPS cells grown for a few days on Matrigel-coated chamber slides. Cells fixed in 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 for 15 minutes at room temperature. Cells were probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 overnight at 4°C, washed with PBST, and incubated with a FITC-conjugated secondary antibody at a dilution of 1:100 for 1 hour at room temperature. Nuclei (blue) were stained with DAPI and cells were analyzed by fluorescence microscopy at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunofluorescent analysis of Sox2 (green) in H9 embryonic stem cells grown for a few days on Matrigel-coated chamber slides. Cells fixed in 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 for 15 minutes at room temperature. Cells were probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 overnight at 4°C, washed with PBST, and incubated with a FITC-conjugated secondary antibody at a dilution of 1:100 for 1 hour at room temperature. Nuclei (blue) were stained with DAPI and cells were analyzed by fluorescence microscopy at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
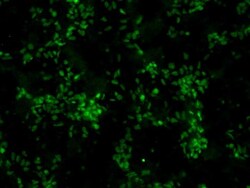
- Experimental details
- Immunofluorescent analysis of Sox2 (green) in human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). The cells were fixed and permeabilized using Image-IT® Fixation/Permeabilization kit (Product # R37602), and blocked with blocking buffer included the kit for one hour at room temperature. Cells were stained with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 in blocking buffer for 3 hours at room temperature, and then incubated with an DyLight 488- conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:250 for 1 hour at room temperature. Image was taken on an EVOS® FLoid® Cell Imaging Station at 10X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
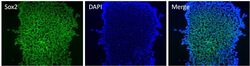
- Experimental details
- Immunofluorescent analysis of Sox2 (green) in H9 embryonic stem cells grown for a few days on Matrigel-coated chamber slides. Cells fixed in 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 for 15 minutes at room temperature. Cells were probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 overnight at 4°C, washed with PBST, and incubated with a FITC-conjugated secondary antibody at a dilution of 1:100 for 1 hour at room temperature. Nuclei (blue) were stained with DAPI and cells were analyzed by fluorescence microscopy at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
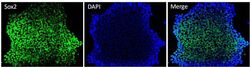
- Experimental details
- Immunofluorescent analysis of Sox2 (green) in HEL 11.4 induced IPS cells grown for a few days on Matrigel-coated chamber slides. Cells fixed in 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 for 15 minutes at room temperature. Cells were probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 overnight at 4°C, washed with PBST, and incubated with a FITC-conjugated secondary antibody at a dilution of 1:100 for 1 hour at room temperature. Nuclei (blue) were stained with DAPI and cells were analyzed by fluorescence microscopy at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
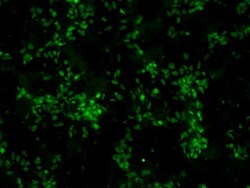
- Experimental details
- Immunofluorescent analysis of Sox2 (green) in human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). The cells were fixed and permeabilized using Image-IT® Fixation/Permeabilization kit (Product # R37602), and blocked with blocking buffer included the kit for one hour at room temperature. Cells were stained with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 in blocking buffer for 3 hours at room temperature, and then incubated with an DyLight 488- conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:250 for 1 hour at room temperature. Image was taken on an EVOS® FLoid® Cell Imaging Station at 10X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
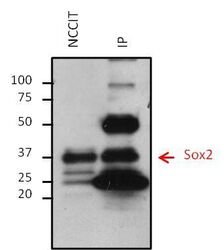
- Experimental details
- Immunoprecipitation of Sox2 was performed on NCCIT cells. Antigen-antibody complexes were formed by incubating 500 µg of NCCIT whole cell lysate with 5 µg of a Sox2 monoclonal antibody (Product # MA1-014) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Plus Agarose (Product # 20423), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Eluted sample and 25 µg of NCCIT whole cell lysate (loading control) were resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBS-0.1%Tween-20 for at least 1 hour. The membrane was probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:1000 overnight rotating at 4°C, washed in TBST, and probed with a goat anti-mouse IgG-HRP secondary antibody (Product # 32430) at a dilution of 1:10,000 for at least 1 hour. Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34075).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
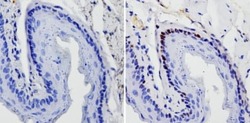
- Experimental details
- Immunohistochemistry analysis of SOX2 showing staining in the nucleus of paraffin-treated mouse esophagus tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SOX2 monoclonal antibody (Product # MA1-014) diluted by 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
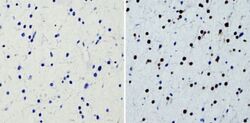
- Experimental details
- Immunohistochemistry analysis of SOX2 showing staining in the nucleus of paraffin-treated human glioma tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SOX2 monoclonal antibody (Product # MA1-014) diluted by 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
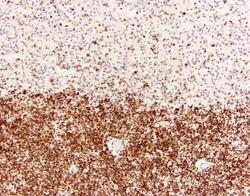
- Experimental details
- Immunohistochemistry was performed on deparaffinized canine fetal brain tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH 6.0) buffer for 20 minutes at 95°C. Following antigen retrieval tissues were blocked with Avidin/Biotin Blocking kit (Product # 00-4303) at room temperature and then probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:200 for one hour at room temperature. Tissues were washed extensively with TBS + 0.025% Triton X-100 (Product # 28314). Detection was performed using a goat anti-mouse HRP secondary antibody (Product # 31430) at a dilution of 1:500 followed by colorimetric detection using metal enhanced DAB (Product # 34065). Tissues were counterstained with hematoxylin and prepped for mounting. Images were taken on a Zeiss Axiovision microscope at 20X magnification. The bottom layer with a dense staining of Sox2 is in the subependymal plate, where there are large numbers of progenitor cells. The upper layer is neuroparenchyma where cells are differentiated, therefore less staining of Sox2. Note: Data courtesy of Innovators Program.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
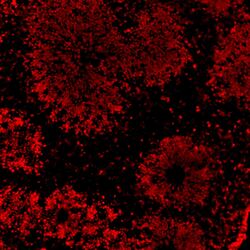
- Experimental details
- Immunofluorescent analysis of SOX2 (red) in human iPSC-derived forebrain organoids derived at Day 40. The organoids were fixed with 4% PFA for 1 hour at room temperature, followed by incubation with 30% sucrose solution overnight at 4°C. The organoids were then embedded in OCT and cryosectioned at 5 µm, permeabilized with 0.2% Triton X-100 for 20 min, and blocked with 10% donkey serum in PBS for 30 min at room temperature. Organoid slices were stained with a Mouse SOX2 monoclonal antibody (red; Product # MA1-014) at a dilution of 1:500 in blocking buffer overnight at 4°C, and then incubated with Donkey anti-Mouse Alexa Fluor 568 (Product # A10037) at a dilution of 1:1000 in blocking solution at room temperature for 1 hour. Images were taken at 20X magnification. Scale bar: 50 µm. Data courtesy of Dr. Zhexing Wen at Emory University.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
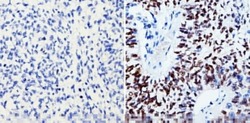
- Experimental details
- Immunohistochemistry analysis of SOX2 showing staining in the nucleus of paraffin-treated human lung squamous carcinoma (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a SOX2 monoclonal antibody (Product # MA1-014) diluted by 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
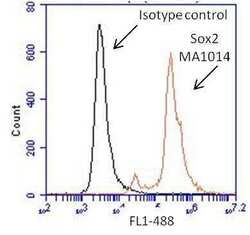
- Experimental details
- Flow cytometry analysis of Sox2 on human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). Cells were fixed, permeabilized and stained with a Sox2 monoclonal antibody (Product # MA1-014, red histogram) or mouse IgG1 isotype control (black histogram) at a 1:100 dilution in 5% BSA. After incubation of the primary antibody for 1 hour on ice, the cells were stained with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:500 for 1 hour on ice. A representative 10,000 cells were acquired for each sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
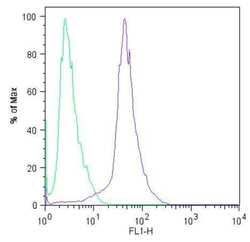
- Experimental details
- Flow cytometric analysis of Sox2 (blue histogram) on HEL 11.4 induced IPS cells. To generate single cells suspensions, colonies were treated with TrypLE cell dissociation enzyme for 5 minutes at 37°C. Cells were incubated with a Sox2 monoclonal antibody (Product # MA1-014) or mouse IgG (green histogram) at a dilution of 1:100 for 1 hour on ice, washed with PBS + 5% fetal calf serum (FACS buffer), and incubated with a FITC-conjugated secondary antibody at a dilution of 1:200 for 30 minutes on ice. Cells were washed with cold FACS buffer, resuspended in 500 µL of FACS buffer containing 10 µL of 4% paraformaldehyde, and analyzed on a flow cytometer.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
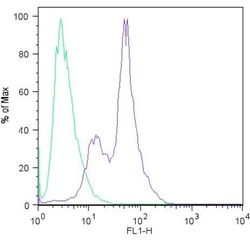
- Experimental details
- Flow cytometric analysis of Sox2 (blue histogram) on H9 embryonic stem cells. To generate single cells suspensions, colonies were treated with TrypLE cell dissociation enzyme for 5 minutes at 37°C. Cells were incubated with a Sox2 monoclonal antibody (Product # MA1-014) or mouse IgG (green histogram) at a dilution of 1:100 for 1 hour on ice, washed with PBS + 5% fetal calf serum (FACS buffer), and incubated with a FITC-conjugated secondary antibody at a dilution of 1:200 for 30 minutes on ice. Cells were washed with cold FACS buffer, resuspended in 500 µL of FACS buffer containing 10 µL of 4% paraformaldehyde, and analyzed on a flow cytometer.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
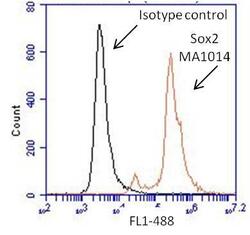
- Experimental details
- Flow cytometry analysis of Sox2 on human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). Cells were fixed, permeabilized and stained with a Sox2 monoclonal antibody (Product # MA1-014, red histogram) or mouse IgG1 isotype control (black histogram) at a 1:100 dilution in 5% BSA. After incubation of the primary antibody for 1 hour on ice, the cells were stained with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:500 for 1 hour on ice. A representative 10,000 cells were acquired for each sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
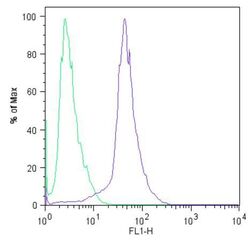
- Experimental details
- Flow cytometric analysis of Sox2 (blue histogram) on HEL 11.4 induced IPS cells. To generate single cells suspensions, colonies were treated with TrypLE cell dissociation enzyme for 5 minutes at 37°C. Cells were incubated with a Sox2 monoclonal antibody (Product # MA1-014) or mouse IgG (green histogram) at a dilution of 1:100 for 1 hour on ice, washed with PBS + 5% fetal calf serum (FACS buffer), and incubated with a FITC-conjugated secondary antibody at a dilution of 1:200 for 30 minutes on ice. Cells were washed with cold FACS buffer, resuspended in 500 µL of FACS buffer containing 10 µL of 4% paraformaldehyde, and analyzed on a flow cytometer.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
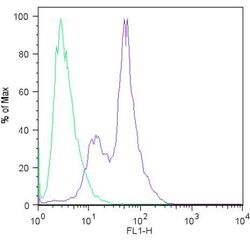
- Experimental details
- Flow cytometric analysis of Sox2 (blue histogram) on H9 embryonic stem cells. To generate single cells suspensions, colonies were treated with TrypLE cell dissociation enzyme for 5 minutes at 37°C. Cells were incubated with a Sox2 monoclonal antibody (Product # MA1-014) or mouse IgG (green histogram) at a dilution of 1:100 for 1 hour on ice, washed with PBS + 5% fetal calf serum (FACS buffer), and incubated with a FITC-conjugated secondary antibody at a dilution of 1:200 for 30 minutes on ice. Cells were washed with cold FACS buffer, resuspended in 500 µL of FACS buffer containing 10 µL of 4% paraformaldehyde, and analyzed on a flow cytometer.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
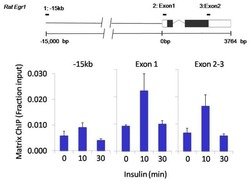
- Experimental details
- Chromatin immunoprecipitation analysis of SOX2 was performed using cross-linked chromatin from 1x10^6 HTC-IR rat hepatoma cells treated with insulin for 0, 10, and 30 minutes. Immunoprecipitation was performed using a multiplex microplate Matrix ChIP assay (see reference for Matrix ChIP protocol: http://www.ncbi.nlm.nih.gov/pubmed/22098709) with 1.0 µL/100 µL well volume of a SOX2 monoclonal antibody (Product # MA1-014). Chromatin aliquots from ~1x10^5 cells were used per ChIP pull-down. Quantitative PCR data were done in quadruplicate using 1 µL of eluted DNA in 2 µL SYBR real-time PCR reactions containing primers to amplify -15kb upstream of the Egr1 gene or exon-1 or exon-2-3 of Egr1. PCR calibration curves were generated for each primer pair from a dilution series of sheared total genomic DNA. Quantitation of immunoprecipitated chromatin is presented as signal relative to the total amount of input chromatin. Results represent the mean +/- SEM for three experiments. A schematic representation of the rat Egr-1 locus is shown above the data where boxes represent exons (black boxes = translated regions, white boxes = untranslated regions), the zigzag line represents an intron, and the straight line represents upstream sequence. Regions amplified by Egr-1 primers are represented by black bars. Data courtesy of the Innovators Program.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
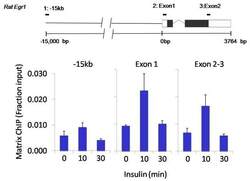
- Experimental details
- Chromatin immunoprecipitation analysis of SOX2 was performed using cross-linked chromatin from 1x10^6 HTC-IR rat hepatoma cells treated with insulin for 0, 10, and 30 minutes. Immunoprecipitation was performed using a multiplex microplate Matrix ChIP assay (see reference for Matrix ChIP protocol: http://www.ncbi.nlm.nih.gov/pubmed/22098709) with 1.0 µL/100 µL well volume of a SOX2 monoclonal antibody (Product # MA1-014). Chromatin aliquots from ~1x10^5 cells were used per ChIP pull-down. Quantitative PCR data were done in quadruplicate using 1 µL of eluted DNA in 2 µL SYBR real-time PCR reactions containing primers to amplify -15kb upstream of the Egr1 gene or exon-1 or exon-2-3 of Egr1. PCR calibration curves were generated for each primer pair from a dilution series of sheared total genomic DNA. Quantitation of immunoprecipitated chromatin is presented as signal relative to the total amount of input chromatin. Results represent the mean +/- SEM for three experiments. A schematic representation of the rat Egr-1 locus is shown above the data where boxes represent exons (black boxes = translated regions, white boxes = untranslated regions), the zigzag line represents an intron, and the straight line represents upstream sequence. Regions amplified by Egr-1 primers are represented by black bars. Data courtesy of the Innovators Program.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
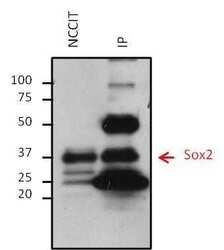
- Experimental details
- Immunoprecipitation of Sox2 was performed on NCCIT cells. Antigen-antibody complexes were formed by incubating 500 µg of NCCIT whole cell lysate with 5 µg of a Sox2 monoclonal antibody (Product # MA1-014) overnight on a rocking platform at 4øC. The immune complexes were captured on 50 µL Protein A/G Plus Agarose (Product # 20423), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Eluted sample and 25 µg of NCCIT whole cell lysate (loading control) were resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBS-0.1%Tween-20 for at least 1 hour. The membrane was probed with a Sox2 monoclonal antibody (Product # MA1-014) at a dilution of 1:1000 overnight rotating at 4øC, washed in TBST, and probed with a goat anti-mouse IgG-HRP secondary antibody (Product # 32430) at a dilution of 1:10,000 for at least 1 hour. Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34075).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
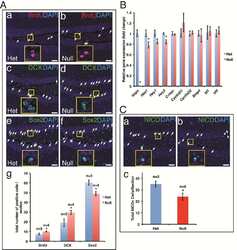
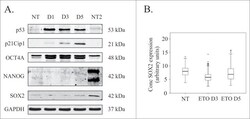
- Figure 3. Analysis of pluripotency transcription factors in response to ETO treatment in PA-1 cells. ( A ) Protein expression of p53, p21Cip1, OCT4A, SOX2 and NANOG were analyzed by immunoblotting at indicated time points after ETO-treatment. GAPDH was used as a loading control NT2 cells were used as a positive control for pluripotency genes OCT4A, SOX2 and NANOG. p53 and p2Cip1 protein expression was upregulated in the DNA damage response. An upregulation of the OCT4A protein was detected from day 1 post ETO treatment, while SOX2 remained low and unchanged post ETO expression. NANOG was not detected in NT or ETO treated PA-1 cells. Results are representative of 3 independent experiments. ( B ) Semi-automatic image cytometry of SOX2 expression was performed on the nuclei of individual cells. Box-plots indicate the heterogeneity and expression levels measured. The data show that SOX2 remains unresponsive to ETO-treatment.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
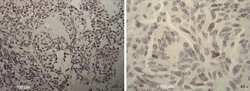
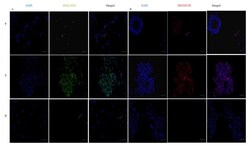
- Figure 2 Immunohistochemical analysis of SOX-2 positive cells in five cases of pneumocytoma of the lung. Tissue sections were stained with anti-SOX-2 antibody to detect the positivity in these five different cases of pneumocytoma of the lung. (A-O) Case 1, (B-P) Case 2, (C-Q) Case 3, (D-R) Case 4, (E-S) Case 5. Semi-quantitative method was used to assess the SOX-2 positivity of the tumor cells: 0 (25-50% positive), 3 (>50-75% positive), and 4 (>75% positive). Representative images of the five cases are shown at 10, 20, and 40x magnification. Isotype control for SOX-2 antibody was shown (F, N, T) . Scale bar = 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
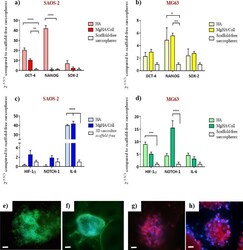
- Figure 7 Gene expression and protein immunofluorescence analysis of 3D sarcospheres scaffold-based models. Relative quantification of OCT-4, NANOG and SOX-2, stemness marker genes, SAOS-2 ( a ) and MG63 ( b ). Relative quantification of HIF-1a, NOTCH-1 and IL-6, CSCs niche-related genes, SAOS-2 ( c ) and MG63 ( d ). The graphs show the fold change expression of the genes relative to the scaffold-free sarcospheres at day 10 of culture (mean +- standard error; * p value
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
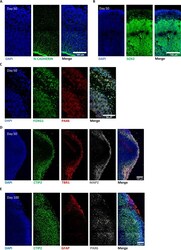
- Fig. 6 Evaluation of cellular identity and morphology in 3D brain organoid. A Representative immunostaining showing the presence of a N-CADHERIN-positive (green) germinal layer in correspondence of the apical membrane of the ventricular zone layer in a FMRP-WT brain organoid at day 50. B , C Representative images revealing forebrain progenitor markers in developing cortical plates within FMRP-WT brain organoid at day 50: B SOX2 (green), DAPI (blue); C PAX6 (red), FOXG1 (green) and DAPI (blue). D Representative staining of early-born deep-layer cortical neurons positive for TBR1 (red), CTIP2 (green) markers, and pan-neuronal MAP2 marker (white) in a FMRP-WT brain organoid at day 50. E Representative images of a cortical plate from a FMRP-WT brain organoid at day 100 revealing heterogeneous cellular population, labeled for specific cortical neuronal marker (CTIP2, green), astrocytic marker (GFAP, red), and neuronal progenitor marker (PAX6, white). Nuclei were stained with DAPI (blue). Scale bar: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
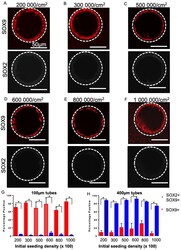
- Figure 6 SOX2-SOX9 status for a range of different seeding densities. Fluorescent micrographs of day 17 lung progenitors seeded for 8 days in 100 um tubes and stained for SOX9 (red) and SOX2 (white) are single positive for SOX9 in 200,000 cells/cm 2 ( A ), 300,000 cells/cm 2 ( B ), 500,000 cells/cm 2 ( C ), 600,000 cells/cm 2 ( D ), 800,000 cells/cm 2 ( E ), 1,000,000 cells/cm 2 ( F ). Bar charts showing quantification of the percentage of SOX9 + cells and the percentage of SOX9 + SOX2 + cells in 100 um tubes ( G ) and 1400 um tubes ( H ) for a range of seeding densities. A significant increase in SOX9 single positive cells and a significant decrease in SOX2 + SOX9 + was observed at all densities in 100-micron diameter tubes; * p < 0.05. Bar, 50 um; the dotted white line approximates the PDMS border; n = 3.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Expression of stemness markers: immunocytochemistry images of ( A ) Sox2 primary antibody stained with FITC and counterstained with DAPI; ( B ) Nestin-primary antibody stained with PE and counterstained with DAPI. The scale bar indicates 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
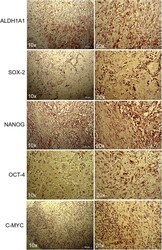
- Experimental details
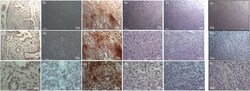
- Figure 3 Immunohistochemistry of lung myofibroblastic tumor. Representative immunohistochemical staining of ALDH1A1, SOX2, NANOG, OCT-4, and c-MYC stem cell markers on a myofibroblastic tumor. Images were shown at 10x and 20x magnification.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry