MA5-14260
antibody from Invitrogen Antibodies
Targeting: CDKN2A
ARF, CDK4I, CDKN2, CMM2, INK4, INK4a, MLM, MTS1, p14, p14ARF, p16, p16INK4a, p19, p19Arf
Antibody data
- Antibody Data
- Antigen structure
- References [26]
- Comments [0]
- Validations
- Western blot [3]
- Immunocytochemistry [2]
- Immunoprecipitation [1]
- Other assay [3]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-14260 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- p14ARF Monoclonal Antibody (14P02 (DCS-240))
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA5-14260 targets p14ARF/p16beta in ICC/IF, IP, and WB applications and shows reactivity with Human samples. The MA5-14260 immunogen is recombinant p14ARF protein.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 14P02 (DCS-240)
- Vial size
- 500 μL
- Concentration
- 0.2 mg/mL
- Storage
- 4°C
Submitted references dCas9 fusion to computer-designed PRC2 inhibitor reveals functional TATA box in distal promoter region.
Nuclear MET requires ARF and is inhibited by carbon nanodots through binding to phospho-tyrosine in prostate cancer.
MMP7 interacts with ARF in nucleus to potentiate tumor microenvironments for prostate cancer progression in vivo.
MDM2-mediated degradation of p14ARF: a novel mechanism to control ARF levels in cancer cells.
A patient with a noninvasive mucinous ovarian borderline tumor presenting with late pleural metastases.
Mimicking p14ARF phosphorylation influences its ability to restrain cell proliferation.
ARF represses androgen receptor transactivation in prostate cancer.
p16INK4A-silencing augments DNA damage-induced apoptosis in cervical cancer cells.
Antiviral action of the tumor suppressor ARF.
Bak functionally complements for loss of Bax during p14ARF-induced mitochondrial apoptosis in human cancer cells.
Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo.
Ebp1 is a dsRNA-binding protein associated with ribosomes that modulates eIF2alpha phosphorylation.
Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways.
p14ARF, a prognostic predictor in HPV-negative vulvar carcinoma.
DNA damage disrupts the p14ARF-B23(nucleophosmin) interaction and triggers a transient subnuclear redistribution of p14ARF.
p14ARF induces G2 cell cycle arrest in p53- and p21-deficient cells by down-regulating p34cdc2 kinase activity.
p14ARF expression in invasive breast cancers and ductal carcinoma in situ--relationships to p53 and Hdm2.
EBP1 is a nucleolar growth-regulating protein that is part of pre-ribosomal ribonucleoprotein complexes.
Immortalization of human mammary epithelial cells is associated with inactivation of the p14ARF-p53 pathway.
Different requirements for the cytostatic and apoptotic effects of type I interferons. Induction of apoptosis requires ARF but not p53 in osteosarcoma cell lines.
Myc-ARF (alternate reading frame) interaction inhibits the functions of Myc.
Nitric oxide induces phosphorylation of p53 and impairs nuclear export.
A melanoma-predisposing germline CDKN2A mutation with functional significance for both p16 and p14ARF.
A melanoma-predisposing germline CDKN2A mutation with functional significance for both p16 and p14ARF.
Aberrant expression of tumor suppressor proteins in the Ewing family of tumors.
p14ARF silencing by promoter hypermethylation mediates abnormal intracellular localization of MDM2.
Levy S, Somasundaram L, Raj IX, Ic-Mex D, Phal A, Schmidt S, Ng WI, Mar D, Decarreau J, Moss N, Alghadeer A, Honkanen H, Sarthy J, Vitanza A, Hawkins RD, Mathieu J, Wang Y, Baker D, Bomsztyk K, Ruohola-Baker H
Cell reports 2022 Mar 1;38(9):110457
Cell reports 2022 Mar 1;38(9):110457
Nuclear MET requires ARF and is inhibited by carbon nanodots through binding to phospho-tyrosine in prostate cancer.
Xie Y, Fan H, Lu W, Yang Q, Nurkesh A, Yeleussizov T, Maipas A, Lu J, Manarbek L, Chen Z, Benassi E
Oncogene 2019 Apr;38(16):2967-2983
Oncogene 2019 Apr;38(16):2967-2983
MMP7 interacts with ARF in nucleus to potentiate tumor microenvironments for prostate cancer progression in vivo.
Xie Y, Lu W, Liu S, Yang Q, Goodwin JS, Sathyanarayana SA, Pratap S, Chen Z
Oncotarget 2016 Jul 26;7(30):47609-47619
Oncotarget 2016 Jul 26;7(30):47609-47619
MDM2-mediated degradation of p14ARF: a novel mechanism to control ARF levels in cancer cells.
Vivo M, Matarese M, Sepe M, Di Martino R, Festa L, Calabrò V, La Mantia G, Pollice A
PloS one 2015;10(2):e0117252
PloS one 2015;10(2):e0117252
A patient with a noninvasive mucinous ovarian borderline tumor presenting with late pleural metastases.
Simons M, Nagtegaal ID, Overbeek LI, Flucke U, Massuger LF, Bulten J
International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 2015 Mar;34(2):143-50
International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 2015 Mar;34(2):143-50
Mimicking p14ARF phosphorylation influences its ability to restrain cell proliferation.
Vivo M, Ranieri M, Sansone F, Santoriello C, Calogero RA, Calabrò V, Pollice A, La Mantia G
PloS one 2013;8(1):e53631
PloS one 2013;8(1):e53631
ARF represses androgen receptor transactivation in prostate cancer.
Lu W, Xie Y, Ma Y, Matusik RJ, Chen Z
Molecular endocrinology (Baltimore, Md.) 2013 Apr;27(4):635-48
Molecular endocrinology (Baltimore, Md.) 2013 Apr;27(4):635-48
p16INK4A-silencing augments DNA damage-induced apoptosis in cervical cancer cells.
Lau WM, Ho TH, Hui KM
Oncogene 2007 Sep 6;26(41):6050-60
Oncogene 2007 Sep 6;26(41):6050-60
Antiviral action of the tumor suppressor ARF.
García MA, Collado M, Muñoz-Fontela C, Matheu A, Marcos-Villar L, Arroyo J, Esteban M, Serrano M, Rivas C
The EMBO journal 2006 Sep 20;25(18):4284-92
The EMBO journal 2006 Sep 20;25(18):4284-92
Bak functionally complements for loss of Bax during p14ARF-induced mitochondrial apoptosis in human cancer cells.
Hemmati PG, Güner D, Gillissen B, Wendt J, von Haefen C, Chinnadurai G, Dörken B, Daniel PT
Oncogene 2006 Oct 26;25(50):6582-94
Oncogene 2006 Oct 26;25(50):6582-94
Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo.
Morris EJ, Michaud WA, Ji JY, Moon NS, Rocco JW, Dyson NJ
PLoS genetics 2006 Nov 17;2(11):e196
PLoS genetics 2006 Nov 17;2(11):e196
Ebp1 is a dsRNA-binding protein associated with ribosomes that modulates eIF2alpha phosphorylation.
Squatrito M, Mancino M, Sala L, Draetta GF
Biochemical and biophysical research communications 2006 Jun 9;344(3):859-68
Biochemical and biophysical research communications 2006 Jun 9;344(3):859-68
Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways.
van der Avoort IA, Shirango H, Hoevenaars BM, Grefte JM, de Hullu JA, de Wilde PC, Bulten J, Melchers WJ, Massuger LF
International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 2006 Jan;25(1):22-9
International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 2006 Jan;25(1):22-9
p14ARF, a prognostic predictor in HPV-negative vulvar carcinoma.
Knopp S, Nesland JM, Tropé C, Holm R
American journal of clinical pathology 2006 Aug;126(2):266-76
American journal of clinical pathology 2006 Aug;126(2):266-76
DNA damage disrupts the p14ARF-B23(nucleophosmin) interaction and triggers a transient subnuclear redistribution of p14ARF.
Lee C, Smith BA, Bandyopadhyay K, Gjerset RA
Cancer research 2005 Nov 1;65(21):9834-42
Cancer research 2005 Nov 1;65(21):9834-42
p14ARF induces G2 cell cycle arrest in p53- and p21-deficient cells by down-regulating p34cdc2 kinase activity.
Normand G, Hemmati PG, Verdoodt B, von Haefen C, Wendt J, Güner D, May E, Dörken B, Daniel PT
The Journal of biological chemistry 2005 Feb 25;280(8):7118-30
The Journal of biological chemistry 2005 Feb 25;280(8):7118-30
p14ARF expression in invasive breast cancers and ductal carcinoma in situ--relationships to p53 and Hdm2.
Vestey SB, Sen C, Calder CJ, Perks CM, Pignatelli M, Winters ZE
Breast cancer research : BCR 2004;6(5):R571-85
Breast cancer research : BCR 2004;6(5):R571-85
EBP1 is a nucleolar growth-regulating protein that is part of pre-ribosomal ribonucleoprotein complexes.
Squatrito M, Mancino M, Donzelli M, Areces LB, Draetta GF
Oncogene 2004 May 27;23(25):4454-65
Oncogene 2004 May 27;23(25):4454-65
Immortalization of human mammary epithelial cells is associated with inactivation of the p14ARF-p53 pathway.
Shamanin VA, Androphy EJ
Molecular and cellular biology 2004 Mar;24(5):2144-52
Molecular and cellular biology 2004 Mar;24(5):2144-52
Different requirements for the cytostatic and apoptotic effects of type I interferons. Induction of apoptosis requires ARF but not p53 in osteosarcoma cell lines.
Sandoval R, Xue J, Pilkinton M, Salvi D, Kiyokawa H, Colamonici OR
The Journal of biological chemistry 2004 Jul 30;279(31):32275-80
The Journal of biological chemistry 2004 Jul 30;279(31):32275-80
Myc-ARF (alternate reading frame) interaction inhibits the functions of Myc.
Datta A, Nag A, Pan W, Hay N, Gartel AL, Colamonici O, Mori Y, Raychaudhuri P
The Journal of biological chemistry 2004 Aug 27;279(35):36698-707
The Journal of biological chemistry 2004 Aug 27;279(35):36698-707
Nitric oxide induces phosphorylation of p53 and impairs nuclear export.
Schneiderhan N, Budde A, Zhang Y, Brüne B
Oncogene 2003 May 15;22(19):2857-68
Oncogene 2003 May 15;22(19):2857-68
A melanoma-predisposing germline CDKN2A mutation with functional significance for both p16 and p14ARF.
Hashemi J, Lindström MS, Asker C, Platz A, Hansson J, Wiman KG
Cancer letters 2002 Jun 28;180(2):211-21
Cancer letters 2002 Jun 28;180(2):211-21
A melanoma-predisposing germline CDKN2A mutation with functional significance for both p16 and p14ARF.
Hashemi J, Lindström MS, Asker C, Platz A, Hansson J, Wiman KG
Cancer letters 2002 Jun 28;180(2):211-21
Cancer letters 2002 Jun 28;180(2):211-21
Aberrant expression of tumor suppressor proteins in the Ewing family of tumors.
Maitra A, Roberts H, Weinberg AG, Geradts J
Archives of pathology & laboratory medicine 2001 Sep;125(9):1207-12
Archives of pathology & laboratory medicine 2001 Sep;125(9):1207-12
p14ARF silencing by promoter hypermethylation mediates abnormal intracellular localization of MDM2.
Esteller M, Cordon-Cardo C, Corn PG, Meltzer SJ, Pohar KS, Watkins DN, Capella G, Peinado MA, Matias-Guiu X, Prat J, Baylin SB, Herman JG
Cancer research 2001 Apr 1;61(7):2816-21
Cancer research 2001 Apr 1;61(7):2816-21
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
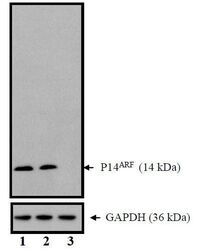
- Western blot of p14ARF/p16beta using p14ARF/p16beta Monoclonal Antibody (Product # MA5-14260) on HeLa Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Western blot analysis of CDKN2A (p14ARF) was performed with 10 µg of HeLa cells transfected with Transfection Reagent alone (Lane 1), 100nM Non-Targeting control siRNA (Lane 2), or 100nM siRNA against CDKN2A (p14ARF) (Lane 3). Proteins were resolved using a NuPAGE® Novex 4-12% Bis-Tris Gel (Product # NP0322BOX), XCell SureLock Electrophoresis System (Product # EI0002), and a protein size ladder. Proteins were wet transferred to a Pierce Nitrocellulose Membrane (Product # 88025) OR Pierce PVDF Membrane (Product # 88518) and blocked with Pierce Starting Block T20 (PBS) Blocking Buffer (Product # 37539) for 1 hour at room temperature. CDKN2A (p14ARF) was detected at ~ 14 kDa using CDKN2A (p14ARF) Mouse monoclonal antibody (Product # MA5-14260) diluted in Pierce Starting Block T20 (PBS) Blocking Buffer 4°C overnight on a rocking platform. Pierce Goat Anti-Mouse (Product # 31437) HRP-Conjugated Antibodies at a 1:2500 dilution were used and chemiluminescent detection was performed using Pierce Supersignal West Dura Maximum Sensitivity Substrate (Product # 37071). Relative density of the bands normalized to GAPDH (36 kDa). CDKN2A (p14ARF) Antibody (Product # MA5-14260) confirms silencing of CDKN2A (p14ARF) expression.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
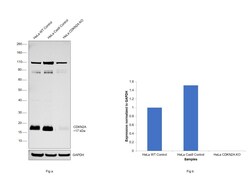
- Experimental details
- Knockout of CDKN2A was achieved by CRISPR-Cas9 genome editing using LentiArray™ Lentiviral sgRNA (Product # A32042) (Assay ID CRISPR697306_LV) and LentiArray Cas9 Lentivirus (Product # A32064). Western blot analysis of CDKN2A was performed by loading 30 µg of HeLa wild type (Lane 1), HeLa CAS9 (Lane 2), HeLa CDKN2A KO (Lane 3) whole cell extracts. The blot was probed with Anti-p14ARF Monoclonal Antibody (14P02 (DCS-240))(Product # MA5-14260) using 2 µg/mL dilution and Goat anti-Mouse IgG (H+L), Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177). Loss of signal upon CRISPR mediated knockout (KO) using the LentiArray™ CRISPR product line confirms that antibody is specific to CDKN2A. An uncharacterized band was observed at ~110 kDa and 70 kDa
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of p14ARF/p16beta (green) showing staining in the nucleus of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a p14ARF/p16beta monoclonal antibody (Product # MA5-14260) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
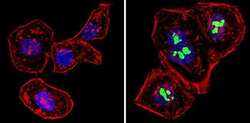
- Main image
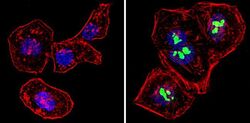
- Experimental details
- Immunofluorescent analysis of p14ARF/p16beta (green) showing staining in the nucleus of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a p14ARF/p16beta monoclonal antibody (Product # MA5-14260) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
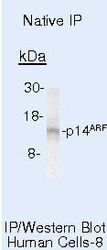
- Experimental details
- Immunoprecipitation of p14ARF/p16beta using p14ARF/p16beta Monoclonal Antibody (Product # MA5-14260) on Native Human HeLa Cells.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of p14ARF/p16beta using p14ARF/p16beta Monoclonal Antibody (Product # MA5-14260) on Native Human HeLa Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
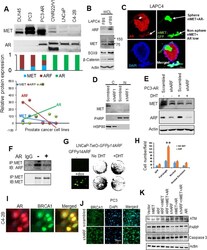
- Experimental details
- Figure 5. Androgen receptor interrupts ARF/MET axis. (A) AR inversely correlates with MET in prostate cancer cell lines. ARF levels were used as reference control based on our previous report ( 20 ). (B) ARF, MET and its signaling proteins beta-Catenin, SOX9 co-upregulation by androgen depletion in LAPC4 cells. LAPC4 cells were cultured in cFBS to deplete androgen for 6 days and regular FBS as control. (C) AR inversely correlates with MET in the prostate sphere. (D) ARF knockdown mediated MET downregulation is cytosolic MET-dependent. C, cytosolic lysates; N, nuclear lysates. (E) ARF knockdown mediated MET downregulation is androgen-dependent and is interrupted by AR activation. (F) AR overexpression and activation disrupts ARF and MET interactions. PC3 cells were transfected by AR and stimulated by DHT at 15nM for 24hrs. (G) ARF inducible expression in LNCaP cells increases androgen independent cell growth. GFP fusion ARF expression is detected by GFP expression by imaging (left panel) and cell growth were detected by crystal violet staining (right panel). (H) Nuclear MET stimulates LNCaP cells migration independent on androgen but mMET dependents on androgen. (I) AR correlates with DNA repair protein BRCA1 in nucleus. (J) ARF knockdown increases BRCA1 nuclear localization. (K) AR, nMET, and ARF regulate ATM and PARP but not caspase 3 investigated by western blot. Data are representative of averages + SD (standard deviation).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
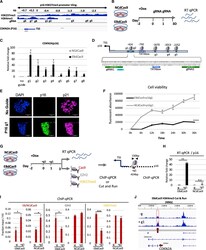
- Experimental details
- Figure 5. EBdCas9 upregulates CDKN2A ( p16 ) expression and compromises cell viability (A) Integrative genomic viewer of CDKN2A H3K27me3 and H3K4me3 promoter tiling. (B) Timeline of EBdCas9 or NCdCas9 induction and gRNA transfection. (C) qRT-PCR analysis of EBdCas9 or NCdCas9 after 3 days of transfection with single gRNA (g1-g8) normalized to beta-actin and relative fold change compared with no guide (-g). (D) TATA boxes/initiator representation with respect to surrounding guide loci using the Element Navigation Tool for detection of core promoter elements. (E) Immunofluorescent imaging of EBdCas9 after 3 days of transfection with single p16 gRNA 1 (+g1) or no transfection (-g). Blue, DAPI; green, p16; far red, p21; scale bar, 100 um. (F) Cell viability of EBdCas9 and NCdCas9 transfected with p16 g1 (+g1) compared with no guide (-g). Time points were analyzed every 6 h using Alamar Blue (n = 3, SEM; *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed t test). (G) EBdCas9 timeline for measuring epigenetic remodeling. (H) qRT-PCR of p16 relative fold change of EBdCas9 and NCdCas9 after 3 days of Dox induction and p16 g1 transfection. Samples were normalized to beta-actin and compared with no guide (-g) of each respective cell line. (I) ChIP-qPCR of induced (+Dox) EBdCas9 and NCdCas9 after 3 days of transfection with p16 g1 RNA (+g1) or no transfection (-g). Antibodies used for ChIP are listed above (Cas9, EZH2, H3K27me3). Normalized to fraction input/H3 (n = 3, SEM; *p < 0.05, **p
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot