PA5-32388
antibody from Invitrogen Antibodies
Targeting: DMD
BMD, DXS142, DXS164, DXS206, DXS230, DXS239, DXS268, DXS269, DXS270, DXS272, MRX85
Antibody data
- Antibody Data
- Antigen structure
- References [12]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-32388 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Dystrophin Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- This antibody is predicted to react with bovine, chicken, canine and porcine based on sequence homology. Heat-mediated antigen retrieval is recommended prior to staining, using a 10mM citrate buffer, pH 6.0, for 10 minutes followed by cooling at room temperature for 20 min. Following antigen retrieval, incubate samples with primary antibody for 10 min at room temperature. A suggested positive control is skeletal muscle tissue.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 500 µL
- Concentration
- Conc. not determined
- Storage
- -20° C, Avoid Freeze/Thaw Cycles
Submitted references Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation.
Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms.
Optogenetic modeling of human neuromuscular circuits in Duchenne muscular dystrophy with CRISPR and pharmacological corrections.
LAT1 Protein Content Increases Following 12 Weeks of Resistance Exercise Training in Human Skeletal Muscle.
Synergist ablation-induced hypertrophy occurs more rapidly in the plantaris than soleus muscle in rats due to different molecular mechanisms.
CRISPR/Cas9 Technology in Restoring Dystrophin Expression in iPSC-Derived Muscle Progenitors.
A gene-edited mouse model of limb-girdle muscular dystrophy 2C for testing exon skipping.
Effect of Ibuprofen on Skeletal Muscle of Dysferlin-Null Mice.
Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease.
Effect of extended postmortem aging and steak location on myofibrillar protein degradation and Warner-Bratzler shear force of beef M. semitendinosus steaks.
Phosphorylation of eIF2α Is a Translational Control Mechanism Regulating Muscle Stem Cell Quiescence and Self-Renewal.
Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth.
Pereyra AS, Lin CT, Sanchez DM, Laskin J, Spangenburg EE, Neufer PD, Fisher-Wellman K, Ellis JM
Molecular metabolism 2022 May;59:101456
Molecular metabolism 2022 May;59:101456
Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms.
Salamone IM, Quattrocelli M, Barefield DY, Page PG, Tahtah I, Hadhazy M, Tomar G, McNally EM
The Journal of clinical investigation 2022 Mar 15;132(6)
The Journal of clinical investigation 2022 Mar 15;132(6)
Optogenetic modeling of human neuromuscular circuits in Duchenne muscular dystrophy with CRISPR and pharmacological corrections.
Paredes-Redondo A, Harley P, Maniati E, Ryan D, Louzada S, Meng J, Kowala A, Fu B, Yang F, Liu P, Marino S, Pourquié O, Muntoni F, Wang J, Lieberam I, Lin YY
Science advances 2021 Sep 10;7(37):eabi8787
Science advances 2021 Sep 10;7(37):eabi8787
LAT1 Protein Content Increases Following 12 Weeks of Resistance Exercise Training in Human Skeletal Muscle.
Roberson PA, Mobley CB, Romero MA, Haun CT, Osburn SC, Mumford PW, Vann CG, Greer RA, Ferrando AA, Roberts MD
Frontiers in nutrition 2020;7:628405
Frontiers in nutrition 2020;7:628405
Synergist ablation-induced hypertrophy occurs more rapidly in the plantaris than soleus muscle in rats due to different molecular mechanisms.
Roberts MD, Mobley CB, Vann CG, Haun CT, Schoenfeld BJ, Young KC, Kavazis AN
American journal of physiology. Regulatory, integrative and comparative physiology 2020 Feb 1;318(2):R360-R368
American journal of physiology. Regulatory, integrative and comparative physiology 2020 Feb 1;318(2):R360-R368
CRISPR/Cas9 Technology in Restoring Dystrophin Expression in iPSC-Derived Muscle Progenitors.
Jin Y, Shen Y, Su X, Weintraub N, Tang Y
Journal of visualized experiments : JoVE 2019 Sep 14;(151)
Journal of visualized experiments : JoVE 2019 Sep 14;(151)
A gene-edited mouse model of limb-girdle muscular dystrophy 2C for testing exon skipping.
Demonbreun AR, Wyatt EJ, Fallon KS, Oosterbaan CC, Page PG, Hadhazy M, Quattrocelli M, Barefield DY, McNally EM
Disease models & mechanisms 2019 Nov 4;13(2)
Disease models & mechanisms 2019 Nov 4;13(2)
Effect of Ibuprofen on Skeletal Muscle of Dysferlin-Null Mice.
Collier AF, Gumerson J, Lehtimäki K, Puoliväli J, Jones JW, Kane MA, Manne S, O'Neill A, Windish HP, Ahtoniemi T, Williams BA, Albrecht DE, Bloch RJ
The Journal of pharmacology and experimental therapeutics 2018 Mar;364(3):409-419
The Journal of pharmacology and experimental therapeutics 2018 Mar;364(3):409-419
Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease.
Kim EY, Page P, Dellefave-Castillo LM, McNally EM, Wyatt EJ
Skeletal muscle 2016;6:32
Skeletal muscle 2016;6:32
Effect of extended postmortem aging and steak location on myofibrillar protein degradation and Warner-Bratzler shear force of beef M. semitendinosus steaks.
Phelps KJ, Drouillard JS, Silva MB, Miranda LD, Ebarb SM, Van Bibber-Krueger CL, O'Quinn TG, Gonzalez JM
Journal of animal science 2016 Jan;94(1):412-23
Journal of animal science 2016 Jan;94(1):412-23
Phosphorylation of eIF2α Is a Translational Control Mechanism Regulating Muscle Stem Cell Quiescence and Self-Renewal.
Zismanov V, Chichkov V, Colangelo V, Jamet S, Wang S, Syme A, Koromilas AE, Crist C
Cell stem cell 2016 Jan 7;18(1):79-90
Cell stem cell 2016 Jan 7;18(1):79-90
Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth.
Gonzalez JM, Camacho LE, Ebarb SM, Swanson KC, Vonnahme KA, Stelzleni AM, Johnson SE
Journal of animal science 2013 Oct;91(10):4797-806
Journal of animal science 2013 Oct;91(10):4797-806
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
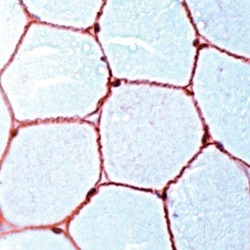
- Experimental details
- Immunohistochemical (paraffin) analysis of Dystrophin using anti-Dystrophin Polyclonal Antibody (Product # PA5-32388) in Skeletal Muscle Cancer Tissue. The recommended dilution for this antibody in immunohistochemistry applications is 1:100.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
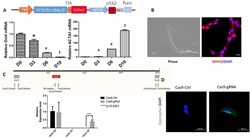
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 3 LAT1 protein increases per skeletal muscle fiber with resistance exercise training but the increase in protein is not located on the membrane. The fold change from PRE, designated as the dashed line at 1.00, for Total LAT1 (A) , Total LAT1 per fiber (B) , Membrane LAT1 (C) , and Membrane LAT1 per fiber (D) protein content for each group using immunohistochemistry. A representative image showing green-stained dystrophin and red-stained LAT1 protein using 20x magnification (E) ; the inset in E is magnified using 40x magnification (F) and the arrows point toward LAT1 protein. The scale bar in both images is 100 mum. *A significant effect. Data are represented as mean +- standard deviation. Sample size for each group is PLA = 10, LEU = 9, and WPC = 9.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
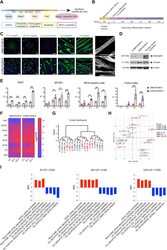
- Experimental details
- Fig. 2. Characterization of DMD and CRISPR-corrected PSC-derived MPCs and myotubes. ( A ) Timeline of the myogenic specification from human ePSCs to MPCs and myotubes with markers for each specific stage. ( B ) Secondary differentiation of MPCs into myotubes. Experiments are analyzed at 0 hours (MPCs in skeletal muscle cell growth medium) and at 24, 48, and 120 hours after switching to secondary differentiation medium. ( C ) Representative immunocytochemistry images of PAX7, MYOD1, dystrophin, MYH (MF20), and titin in DMD-R3381X and CORR-R3381X myogenic cultures at 120 hours of secondary differentiation. Enlarged images of titin demonstrate sarcomere striation. Scale bars, 100 mum. ( D ) Immunoblotting confirms that full-length dystrophin (427 kDa) is restored in CORR-R3381X myogenic cultures. ( E ) Quantification of PAX7-positive, MYOD1-positive, and MF20-positive cells and fusion index during secondary differentiation at 0, 24, 48, and 120 hours in DMD-R3381X and CORR-R3381X cultures. Values are means +- SD. n = 6. Two-way analysis of variance (ANOVA) and Sidak's multiple comparisons test, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. ( F ) Heatmap of normalized gene expression levels as log 2 transformed RPKM (reads per kilobase per million mapped reads) of myogenic markers in DMD-R3381X and CORR-R3381X cells at 0, 24, and 120 hours of secondary differentiation. ( G ) Unsupervised cluster dendrogram of DMD-R3381X and CORR-R3381X transcriptomes at 0, 24, and 120 hours of
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
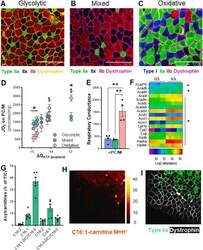
- Experimental details
- Muscle fiber type composition is a major determinant in fatty acid-supported mitochondrial respiration. (A), (B), (C) Representative imaging of muscle fiber typing via Myosin Heavy Chain (MyHC) immunohistochemistry in predominately glycolytic (EDL), mixed (TA), and oxidative (Soleus) muscles. Magnification 20X. Scale bar 200 mum. (D) Mitochondrial respiration ( JO 2 ) across a continuum of ATP free energy (DeltaG ATP ) and (E) respiratory conductance in predominantly glycolytic, mixed, and oxidative muscles energized with palmitoylcarnitine/malate (PC/M). (F) Heatmap of abundance of proteins involved in mitochondrial fatty acid oxidation in glycolytic (EDL) and oxidative (SOL) muscles. (G) Relative abundance of long-chain acylcarnitines (LCACs) in mixed TA muscle. (H) Digital reconstitution of mass spectroscopy-based lipid scanning (nano-DESI) of palmitoleyl-carnitine (C16:1) in mixed TA muscle and (I) immunostaining for myosin heavy chain type 2A (green) and dystrophin (white) on consecutive muscle section. For (D), (E), and (G), data is presented as Mean +- SEM; n = 3-6 and *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
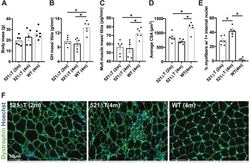
- Fig. 3. Reduced mass and myofiber area in Sgcg 521DeltaT mice. (A) Body mass was not significantly different among genotypes. (B) Gluteus/hamstring (GH) muscle mass normalized to tibia length was reduced in 521DeltaT mice compared with controls. (C) The total mass from muscle groups normalized to tibia length was significantly reduced in 521DeltaT mice compared with WT controls (quadriceps, gluteus/hamstring, triceps, diaphragm, heart, tibialis anterior muscle groups combined). (D) Average myofiber cross-sectional area (CSA) was significantly smaller in 521DeltaT mice than in WT controls. (E) Percentage of myofibers with >1 internal nuclei was increased in 521DeltaT muscle, with 4-month-old 521DeltaT myofibers having the most internal nuclei. (F) Representative images of 521DeltaT and WT muscle. Anti-dystrophin (green) outlined myofibers. Hoechst (blue) demonstrated nuclei. Data are mean+-s.e.m. * P =5 mice per group). Scale bars: 50 um.
 Explore
Explore Validate
Validate Learn
Learn Immunohistochemistry
Immunohistochemistry