Antibody data
- Antibody Data
- Antigen structure
- References [12]
- Comments [0]
- Validations
- Immunohistochemistry [3]
- Other assay [7]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-22863 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Sarcomeric alpha Actinin Monoclonal Antibody (EA-53)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA1-22863 detects sarcomeric alpha Actinin in bovine, feline, chicken, fish, goat, hamster, lizard, mouse porcine, rabbit, rat, ovine, snake, and Xenopus laevis samples.
- Reactivity
- Human, Mouse, Rat, Rabbit, Zebrafish
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- EA-53
- Vial size
- 500 μL
- Concentration
- Conc. Not Determined
- Storage
- 4°C
Submitted references Cardiac ultrastructure inspired matrix induces advanced metabolic and functional maturation of differentiated human cardiomyocytes.
Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells.
Pre-clinical dose-escalation studies establish a therapeutic range for U7snRNA-mediated DMD exon 2 skipping.
Human Cardiac Organoids for Modeling Genetic Cardiomyopathy.
Large-scale spontaneous self-organization and maturation of skeletal muscle tissues on ultra-compliant gelatin hydrogel substrates.
On-site fabrication of Bi-layered adhesive mesenchymal stromal cell-dressings for the treatment of heart failure.
Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis.
Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function.
An internal promoter underlies the difference in disease severity between N- and C-terminal truncation mutations of Titin in zebrafish.
Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery.
Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes.
Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment.
Afzal J, Liu Y, Du W, Suhail Y, Zong P, Feng J, Ajeti V, Sayyad WA, Nikolaus J, Yankova M, Deymier AC, Yue L, Kshitiz
Cell reports 2022 Jul 26;40(4):111146
Cell reports 2022 Jul 26;40(4):111146
Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells.
Ng WH, Johnston EK, Tan JJ, Bliley JM, Feinberg AW, Stolz DB, Sun M, Wijesekara P, Hawkins F, Kotton DN, Ren X
eLife 2022 Jan 12;11
eLife 2022 Jan 12;11
Pre-clinical dose-escalation studies establish a therapeutic range for U7snRNA-mediated DMD exon 2 skipping.
Simmons TR, Vetter TA, Huang N, Vulin-Chaffiol A, Wein N, Flanigan KM
Molecular therapy. Methods & clinical development 2021 Jun 11;21:325-340
Molecular therapy. Methods & clinical development 2021 Jun 11;21:325-340
Human Cardiac Organoids for Modeling Genetic Cardiomyopathy.
Filippo Buono M, von Boehmer L, Strang J, Hoerstrup SP, Emmert MY, Nugraha B
Cells 2020 Jul 20;9(7)
Cells 2020 Jul 20;9(7)
Large-scale spontaneous self-organization and maturation of skeletal muscle tissues on ultra-compliant gelatin hydrogel substrates.
Jensen JH, Cakal SD, Li J, Pless CJ, Radeke C, Jepsen ML, Jensen TE, Dufva M, Lind JU
Scientific reports 2020 Aug 6;10(1):13305
Scientific reports 2020 Aug 6;10(1):13305
On-site fabrication of Bi-layered adhesive mesenchymal stromal cell-dressings for the treatment of heart failure.
Kobayashi K, Ichihara Y, Sato N, Umeda N, Fields L, Fukumitsu M, Tago Y, Ito T, Kainuma S, Podaru M, Lewis-McDougall F, Yamahara K, Uppal R, Suzuki K
Biomaterials 2019 Jul;209:41-53
Biomaterials 2019 Jul;209:41-53
Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis.
Yang C, Xu Y, Yu M, Lee D, Alharti S, Hellen N, Ahmad Shaik N, Banaganapalli B, Sheikh Ali Mohamoud H, Elango R, Przyborski S, Tenin G, Williams S, O'Sullivan J, Al-Radi OO, Atta J, Harding SE, Keavney B, Lako M, Armstrong L
Human molecular genetics 2017 Aug 15;26(16):3031-3045
Human molecular genetics 2017 Aug 15;26(16):3031-3045
Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function.
Sharma P, Abbasi C, Lazic S, Teng ACT, Wang D, Dubois N, Ignatchenko V, Wong V, Liu J, Araki T, Tiburcy M, Ackerley C, Zimmermann WH, Hamilton R, Sun Y, Liu PP, Keller G, Stagljar I, Scott IC, Kislinger T, Gramolini AO
Nature communications 2015 Sep 25;6:8391
Nature communications 2015 Sep 25;6:8391
An internal promoter underlies the difference in disease severity between N- and C-terminal truncation mutations of Titin in zebrafish.
Zou J, Tran D, Baalbaki M, Tang LF, Poon A, Pelonero A, Titus EW, Yuan C, Shi C, Patchava S, Halper E, Garg J, Movsesyan I, Yin C, Wu R, Wilsbacher LD, Liu J, Hager RL, Coughlin SR, Jinek M, Pullinger CR, Kane JP, Hart DO, Kwok PY, Deo RC
eLife 2015 Oct 16;4:e09406
eLife 2015 Oct 16;4:e09406
Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery.
Guan X, Mack DL, Moreno CM, Strande JL, Mathieu J, Shi Y, Markert CD, Wang Z, Liu G, Lawlor MW, Moorefield EC, Jones TN, Fugate JA, Furth ME, Murry CE, Ruohola-Baker H, Zhang Y, Santana LF, Childers MK
Stem cell research 2014 Mar;12(2):467-80
Stem cell research 2014 Mar;12(2):467-80
Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes.
Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, Chaudhry HW
Science translational medicine 2014 Feb 19;6(224):224ra27
Science translational medicine 2014 Feb 19;6(224):224ra27
Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment.
Maioli M, Contini G, Santaniello S, Bandiera P, Pigliaru G, Sanna R, Rinaldi S, Delitala AP, Montella A, Bagella L, Ventura C
Drug design, development and therapy 2013;7:1063-73
Drug design, development and therapy 2013;7:1063-73
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry (Paraffin) analysis of Sarcomeric alpha Actinin was performed in formalin-fixed, paraffin-embedded human skeletal muscle tissue using Sarcomeric alpha Actinin Monoclonal Antibody (EA-53) (Product # MA1-22863).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemical (Paraffin) analysis of human skeletal muscle tissue using (Product # MA1-22863) Sarcomeric alpha Actinin Monoclonal Antibody (EA-53).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunohistochemistry (Paraffin) analysis of Sarcomeric alpha Actinin in human skeletal muscle tissue using Sarcomeric alpha Actinin Monoclonal Antibody (EA-53) (Product # MA1-22863).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
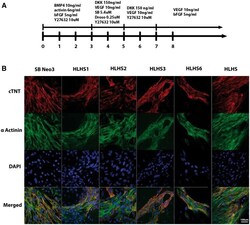
- Figure 2 Differentiation of iPSC lines to cardiomyocytes. ( A ) Overview of the protocol used for differentiation of control and HLHS patient specific iPSC to cardiomyocytes; ( B ) Representative immunocytochemical staining of cardiomyocytes derived from control and HLHS patient specific iPSC lines using antibodies raised against alpha actinin and cardiac troponin. One clone from each patient and unaffected control is shown; however similar data were obtained for the second clone. Scale bar = 100 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
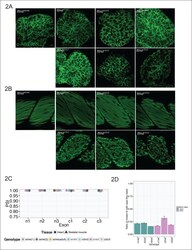
- Figure 2. C-terminal ttna truncations result in a severe skeletal muscle phenotype while N-terminal truncations are indistinguishable from wild-type. Fixed heart ( A ) and skeletal muscle ( B ) samples of 72 hpf ttna wt/wt , ttna n/n and ttna c/c mutant embryos were analyzed by immunostaining for alpha-actinin, which highlights Z-disc architecture. The cardiac sarcomere was disarrayed in all mutants. However, in skeletal muscle ttna c/c mutants demonstrated severe sarcomeric disarray while ttna n/n mutants retained sarcomeric architecture. Scale bar: 10 umum. ( C ) All targeted TTN exons are constitutive (i.e. not alternatively spliced) in both cardiac and skeletal muscle. PSI values computed for each mutated exon using RNA-Seq data for dissected hearts and trunk skeletal muscle for various mutant genotypes at 72 hpf. Wild-type fish were analyzed at both 72 hpf and adulthood. Analysis was limited to samples with a sufficient number of exon-exon junction reads to accurately estimate PSI ( Pervouchine et al., 2013 ). ( D ) Nonsense-mediated decay reduces mutant transcript levels to ~20-25% of wild-type, but does not vary substantially across mutations. Targeted RNA-Seq was used to determine the ratio of reads derived from the mutant allele vs. the wild-type allele in ttna n/wt and ttna c/wt heterozygote mutants, which serves as an estimate of nonsense-mediated decay efficiency. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
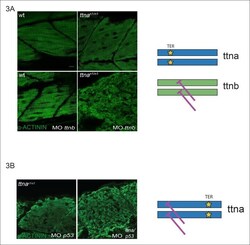
- Figure 3. C-terminal ttna truncations do not act as dominant negatives. ( A ) Knockdown of ttnb by morpholino injection worsens skeletal muscle sarcomeric architecture in ttna n/n mutants. Fixed skeletal muscle samples of 72 hpf ttna n1/n1 mutant embryos were analyzed by immunostaining for alpha-actinin. (right) Cartoon representation of ttna and ttnb proteins, with a premature N-terminal stop codon in ttna (gold star). The purple lines indicate morpholino disruption of the ttnb transcript. Scale bar: 10 umum. ( B ) Knockdown of ttna by morpholino injection does not rescue skeletal muscle architecture in ttna c1/c1 mutants. ttna c1/c1 embyros were injected with a ttna splice-site morpholino to the exon 4-intron 4 junction at the 1-2 cell stage and embryos were examined at 72 hpf. At this morpholino dose, knockdown efficacy was estimated at close to 80% and nearly complete cessation of cardiac contraction was achieved in >90% of wild-type embryos (data not shown). Immunostaining for alpha-actinin revealed no improvement in skeletal muscle architecture. (right) Cartoon representation of mutant ttna proteins, with a premature C-terminal stop codon (gold star). The purple lines indicate disruption of the ttna transcript using a morpholino that is expected to introduce an N-terminal truncation upstream of the C-terminal truncation. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
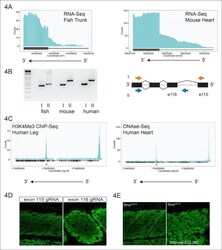
- Figure 4. An internal promoter at the distal I-band explains phenotypic differences between N- an C-terminal ttna mutants. ( A ) RNA-Seq data from 72 hpf zebrafish trunk (left) and E12.5 mouse hearts (right) depicting accumulation of reads within the intron, upstream of the orthologous exons 116 (zebrafish) and 223 (mouse). The Ttn gene is on the negative strand and so reads within the upstream intron are shown to the right of the black bar, which indicates the position of the relevant exon. ( B ) Transcription from an alternative Titin promoter occurs in zebrafish, mouse, and human heart. (left) PCR amplification using a primer in the upstream exon or an internal primer at or near the internal TSS (as determined by 5'-RACE) as forward primer and a shared reverse primer was performed using cDNA from zebrafish, mouse and human heart. For all three species, products of the expected size were found, supporting transcription from an internal promoter. (right) Cartoon representation of PCR amplification scheme, with exon numbering according to zebrafish transcript. The zebrafish 5' UTR is shorter than that of mouse and human. ( C ) (left) H3K4me3 ChIP-Seq data from human fetal leg muscle (GEO Accession GSM1058781), indicating an active promoter overlies exon 240, which is orthologous to exon 116 in zebrafish. The peak at the far right of the figure represents the conventional human TTN promoter. (right) DNAse-Seq data from human fetal heart (GEO Accession GSM665830) indi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
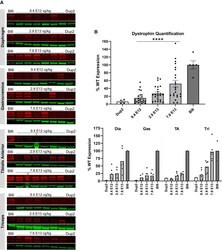
- Experimental details
- Figure 10 Immunoblot analysis demonstrates continued dystrophin expression 12 weeks after i.v. injection of scAAV9.U7.ACCA (A) Immunoblots for dystrophin show a sustained increase in dystrophin protein expression (red) normalized to actinin (green) across four different muscles 23 weeks after treatment. Sample lanes marked with a red X were omitted due to technical issues. (B) Immunoblot quantification confirms a significant increase in dystrophin protein expression with increasing dose. Quantification reported as mean +- 95% CI (shaded region), with individual data points representing individual samples. Quantification reported as mean +- SEM, with individual data points representing individual samples. Statistical analysis was performed on pooled data from all skeletal muscles using one-way ANOVA with a post hoc test for linear trend from left to right; ****p < 0.0001 for trend. Bottom panel displays quantification results broken down by individual muscles.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
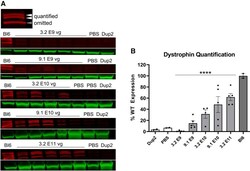
- Experimental details
- Figure 3 Immunoblot analysis following i.m. injection of scAAV9.U7.ACCA in the TA in Dup2 mice (A) Assessment of dystrophin expression by immunoblotting shows a dose-dependent increase in dystrophin protein that is confirmed by quantification of the dystrophin band doublet (red) normalized to actinin (green). (B) Quantification results reported as mean +- SEM, with individual data points representing individual samples. Statistical analysis was performed using one-way ANOVA with a post hoc test for linear trend from left to right; ****p < 0.0001 for trend.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
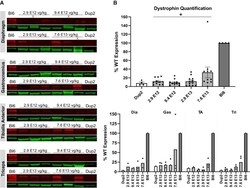
- Experimental details
- Figure 7 Immunoblot analysis following delivery of scAAV9.U7.ACCA confirms dystrophin expression 4 weeks post-injection (A and B) Representative images of immunoblots (A) show increase in dystrophin protein (red) normalized to actinin (green), and quantification (B) confirms a significant increase in dystrophin protein expression with increasing dose. Sample lanes marked with a red X were omitted due to technical issues. Quantification reported as mean +- SEM, with individual data points representing individual samples. Statistical analysis was performed on pooled data from all skeletal muscles using one-way ANOVA with a post hoc test for linear trend from left to right; *p < 0.05 for trend. Lower panel displays quantification results broken down by individual muscles.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry