36-6900
antibody from Invitrogen Antibodies
Targeting: APP
AD1
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [20]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Flow cytometry [1]
- Other assay [30]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 36-6900 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- beta Amyloid Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- For immunohistochemistry in formalin-fixed, paraffin-embedded tissues, digestion with trypsin prior to staining is required.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 0.25 mg/mL
- Storage
- -20°C
Submitted references Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients.
A Cautionary Tale: Endogenous Biotinylated Proteins and Exogenously-Introduced Protein A Cause Antibody-Independent Artefacts in Western Blot Studies of Brain-Derived Proteins.
Autoimmune-Mediated Retinopathy in CXCR5-Deficient Mice as the Result of Age-Related Macular Degeneration Associated Proteins Accumulation.
Nimodipine treatment does not benefit juvenile ferrets with kaolin-induced hydrocephalus.
Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury.
Repetitive Closed-Head Impact Model of Engineered Rotational Acceleration Induces Long-Term Cognitive Impairments with Persistent Astrogliosis and Microgliosis in Mice.
Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma.
Quaternary Structure Defines a Large Class of Amyloid-β Oligomers Neutralized by Sequestration.
Reduced subventricular zone proliferation and white matter damage in juvenile ferrets with kaolin-induced hydrocephalus.
Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause.
Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury.
Memory deficits of British dementia knock-in mice are prevented by Aβ-precursor protein haploinsufficiency.
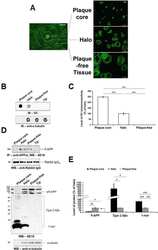
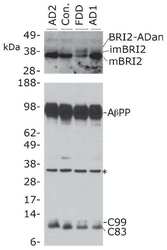
Increased AβPP processing in familial Danish dementia patients.
APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant.
Herpes simplex virus dances with amyloid precursor protein while exiting the cell.
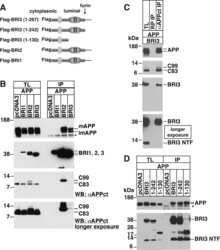
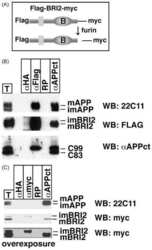
Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles.
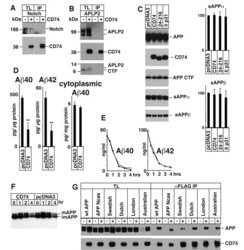
CD74 interacts with APP and suppresses the production of Abeta.
BRI3 inhibits amyloid precursor protein processing in a mechanistically distinct manner from its homologue dementia gene BRI2.
BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate.
The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production.
Farré-Alins V, Palomino-Antolín A, Narros-Fernández P, Lopez-Rodriguez AB, Decouty-Perez C, Muñoz-Montero A, Zamorano-Fernández J, Mansilla-Fernández B, Giner-García J, García-Feijoo P, Sáez-Alegre M, Palpán-Flores AJ, Roda-Frade JM, Carabias CS, Rosa JM, Civantos-Martín B, Yus-Teruel S, Gandía L, Lagares A, Hernández-García BJ, Egea J
Biomedicines 2021 May 25;9(6)
Biomedicines 2021 May 25;9(6)
A Cautionary Tale: Endogenous Biotinylated Proteins and Exogenously-Introduced Protein A Cause Antibody-Independent Artefacts in Western Blot Studies of Brain-Derived Proteins.
Grant MKO, Shapiro SL, Ashe KH, Liu P, Zahs KR
Biological procedures online 2019;21:6
Biological procedures online 2019;21:6
Autoimmune-Mediated Retinopathy in CXCR5-Deficient Mice as the Result of Age-Related Macular Degeneration Associated Proteins Accumulation.
Lennikov A, Saddala MS, Mukwaya A, Tang S, Huang H
Frontiers in immunology 2019;10:1903
Frontiers in immunology 2019;10:1903
Nimodipine treatment does not benefit juvenile ferrets with kaolin-induced hydrocephalus.
Di Curzio DL, Mao X, Baker A, Del Bigio MR
Fluids and barriers of the CNS 2018 May 3;15(1):14
Fluids and barriers of the CNS 2018 May 3;15(1):14
Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury.
Wu J, Vogel T, Gao X, Lin B, Kulwin C, Chen J
Scientific reports 2018 Mar 21;8(1):4935
Scientific reports 2018 Mar 21;8(1):4935
Repetitive Closed-Head Impact Model of Engineered Rotational Acceleration Induces Long-Term Cognitive Impairments with Persistent Astrogliosis and Microgliosis in Mice.
Chen H, Desai A, Kim HY
Journal of neurotrauma 2017 Jul 15;34(14):2291-2302
Journal of neurotrauma 2017 Jul 15;34(14):2291-2302
Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma.
Winston CN, Noël A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D, Wilkins TE, Alikhani AD, Zapple DN, Villapol S, Planel E, Burns MP
The American journal of pathology 2016 Mar;186(3):552-67
The American journal of pathology 2016 Mar;186(3):552-67
Quaternary Structure Defines a Large Class of Amyloid-β Oligomers Neutralized by Sequestration.
Liu P, Reed MN, Kotilinek LA, Grant MK, Forster CL, Qiang W, Shapiro SL, Reichl JH, Chiang AC, Jankowsky JL, Wilmot CM, Cleary JP, Zahs KR, Ashe KH
Cell reports 2015 Jun 23;11(11):1760-71
Cell reports 2015 Jun 23;11(11):1760-71
Reduced subventricular zone proliferation and white matter damage in juvenile ferrets with kaolin-induced hydrocephalus.
Di Curzio DL, Buist RJ, Del Bigio MR
Experimental neurology 2013 Oct;248:112-28
Experimental neurology 2013 Oct;248:112-28
Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause.
Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G, Vadlamudi RK, Brann DW
Brain : a journal of neurology 2013 May;136(Pt 5):1432-45
Brain : a journal of neurology 2013 May;136(Pt 5):1432-45
Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury.
Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW
PloS one 2012;7(4):e34504
PloS one 2012;7(4):e34504
Memory deficits of British dementia knock-in mice are prevented by Aβ-precursor protein haploinsufficiency.
Tamayev R, D'Adamio L
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Apr 18;32(16):5481-5
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Apr 18;32(16):5481-5
Increased AβPP processing in familial Danish dementia patients.
Matsuda S, Tamayev R, D'Adamio L
Journal of Alzheimer's disease : JAD 2011;27(2):385-91
Journal of Alzheimer's disease : JAD 2011;27(2):385-91
APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant.
Tamayev R, Matsuda S, Giliberto L, Arancio O, D'Adamio L
The EMBO journal 2011 May 17;30(12):2501-9
The EMBO journal 2011 May 17;30(12):2501-9
Herpes simplex virus dances with amyloid precursor protein while exiting the cell.
Cheng SB, Ferland P, Webster P, Bearer EL
PloS one 2011 Mar 31;6(3):e17966
PloS one 2011 Mar 31;6(3):e17966
Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles.
Matsuda S, Matsuda Y, Snapp EL, D'Adamio L
Neurobiology of aging 2011 Aug;32(8):1400-8
Neurobiology of aging 2011 Aug;32(8):1400-8
CD74 interacts with APP and suppresses the production of Abeta.
Matsuda S, Matsuda Y, D'Adamio L
Molecular neurodegeneration 2009 Oct 22;4:41
Molecular neurodegeneration 2009 Oct 22;4:41
BRI3 inhibits amyloid precursor protein processing in a mechanistically distinct manner from its homologue dementia gene BRI2.
Matsuda S, Matsuda Y, D'Adamio L
The Journal of biological chemistry 2009 Jun 5;284(23):15815-25
The Journal of biological chemistry 2009 Jun 5;284(23):15815-25
BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate.
Matsuda S, Giliberto L, Matsuda Y, McGowan EM, D'Adamio L
The Journal of neuroscience : the official journal of the Society for Neuroscience 2008 Aug 27;28(35):8668-76
The Journal of neuroscience : the official journal of the Society for Neuroscience 2008 Aug 27;28(35):8668-76
The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production.
Matsuda S, Giliberto L, Matsuda Y, Davies P, McGowan E, Pickford F, Ghiso J, Frangione B, D'Adamio L
The Journal of biological chemistry 2005 Aug 12;280(32):28912-6
The Journal of biological chemistry 2005 Aug 12;280(32):28912-6
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
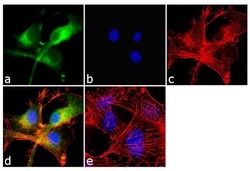
- Experimental details
- Immunofluorescent analysis of beta-Amyloid was performed using 70% confluent log phase U-87 MG cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with beta-Amyloid Rabbit Polyclonal Antibody (Product # 36-6900) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
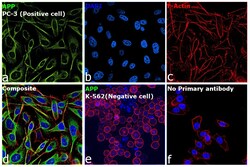
- Experimental details
- Immunofluorescence analysis of beta Amyloid was performed using 70% confluent log phase PC-3 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with beta Amyloid Polyclonal Antibody (Product # 36-6900) at a concentration of 1 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization in PC-3 cells. Panel e represents K-562 cells showing no cytoplasmic staining. Panel f represents control PC-3 cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
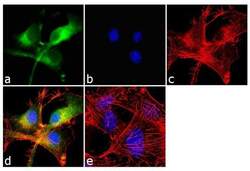
- Experimental details
- Immunofluorescent analysis of beta-Amyloid was performed using 70% confluent log phase U-87 MG cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with beta-Amyloid Rabbit Polyclonal Antibody (Product # 36-6900) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
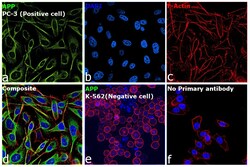
- Experimental details
- Immunofluorescence analysis of beta Amyloid was performed using 70% confluent log phase PC-3 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with beta Amyloid Polyclonal Antibody (Product # 36-6900) at a concentration of 1 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization in PC-3 cells. Panel e represents K-562 cells showing no cytoplasmic staining. Panel f represents control PC-3 cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
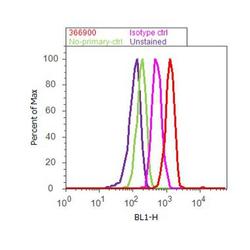
- Experimental details
- Flow cytometry analysis of beta-Amyloid was done on U-87 MG cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with beta-Amyloid Rabbit Polyclonal Antibody (36-6900, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10, 000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
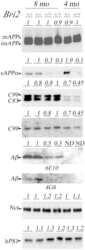
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
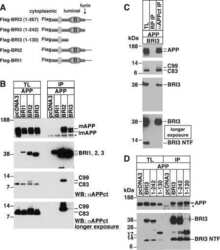
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
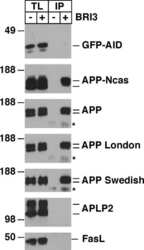
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
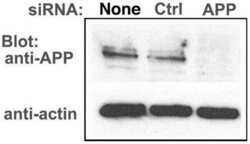
- Figure 6 APP knock-down by siRNA decreases APP protein >90% by Western blotting. HSV1 infected cells were transfected in parallel with either vehicle alone (None), non-silencing RNA (Ctrl) or siRNA against APP (APP). After 48 hr cells were scraped into lysis buffer, and loaded in parallel on a 10% gel for electrophoresis followed by transfer to nitrocellulose. The blot was divided in two horizontally, the top half probed for APP and the lower half for actin, a loading control. Non-silencing siRNA has little effect, while siRNA for APP decreases APP band intensity almost entirely, with no significant effect on actin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
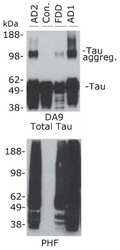
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
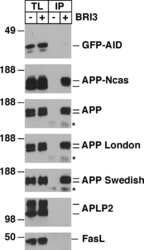
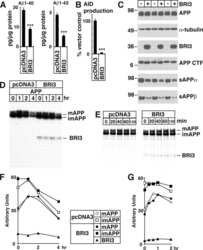
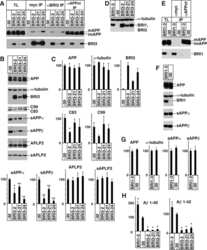
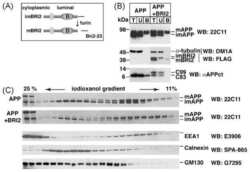
- Figure 2 A) HeLa cells were transfected with pcDNA3 (-) or CD74 (+), together with Notch . The total cell lysates (TL) and FLAG IPs were analyzed as in Figure 1B. Notch was detected with alphamyc antibody. B) HeLa cells were transfected with pcDNA3 (-) or CD74 (+), together with APLP2, and were analyzed as in A. C) Left panels: HEK293APP cells were transfected with the indicated plasmids. The transfected cells were further incubated for 24 hours with fresh media, and secreted sAPPalpha/beta were measured by Westerm blot. The cell lysates were analyzed as in Figure 1B. Right panels: the quantification of sAPPalpha/beta. The levels in pcDNA3 transfected cells were set to 100. D) HEK293APP cells were transfected with pcDNA3 or CD74 and treated as in C. Abeta40 and 42 secreted in the media, and Abeta40 in the lysates were measured with specific ELISA. The amount of Abeta was normalized to the amount of the protein in the total lysates. The asterisks (**) indicate that p values by Student's t test are less than 0.01. E) HeLa cells were transfected with pcDNA3 or CD74, and the media was replaced with fresh media containing synthetic Abeta40 and Abeta42. The media of pcDNA3 (open circles) and CD74 (closed circles) transfected cells were incubated for indicated hours, and the remaining Abeta40 and Abeta42 were measured as in D. F) HeLa cells were transfected with pcDNA3 or FLAG-CD74 together with APP. The cells were pulsed for 30 min with 35 S methionine+cysteine, and chased in
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
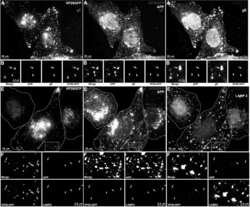
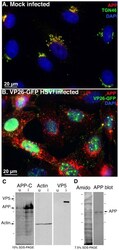
- Figure 2 APP relocalizes in infected cells. (A) APP (red) co-localizes with TGN46 (green) in a compact tuft to one side of the nucleus (DAPI, blue) representing the trans-Golgi network in mock infected cells. (B) In cells infected with VP26-GFP HSV1 (green), APP (red) is distributed in particles throughout the cytoplasm at 7 hr p.i. Nuclei are stained with DAPI (blue). (C) Western blotting of uninfected (u) and infected (i) cells with anti-APP, anti-actin (loading control) and anti-VP5 (viral capsid) demonstrates significant increased amound of APP in infected cells. Actin bands remain similar, and VP5, as expected, is only detected in lanes loaded with infected cell lysate. Note no new APP bands are detected in the infected versus uninfected cell lysates. (D) Isolated virus separated on 7.5% SDS-PAGE and stained with amido black for protein and probed for APP by Western blotting with the same antibodies used for immuno-fluorescence. Note that only the 90-110 kD doublet representing APP is detected by anti-APP, with no additional viral protein bands detected. See also Figure S2 for split channels and Figure S3 for histone staining.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
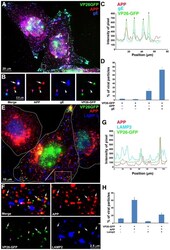
- Experimental details
- Figure 4 Out-going cytoplasmic VP26-GFP particles co-localize with APP and not with LAMP2. Cells were synchronously infected with VP26-GFP HSV1 (green) and fixed at 7 hr p.i.. (A-D) Examples of infected cells stained for gE (blue) and APP (red). In (B) a high magnification of boxed region in (A) is shown. A white arrow indicates one of the particles displaying all three labels (gE, APP and VP26). Particles with APP only (white arrowhead) or gE and VP26 (pink arrowhead) are indicated. (F-H) Examples of infected cells stained for LAMP2 (blue) and APP (red). In (F) a high magnification of the boxed regions in (E) is shown. White arrows indicate APP and gE, pink arrowheads indicate LAMP2, and yellow arrowheads indicate APP alone. (C and G) Intensity profiles along a line (white) drawn across the merged image in (A) and (E). Arrows indicate the superposition of peaks for each channel. (D and H) Histograms showing the percentage of VP26-GFP particles in each category. VP26-GFP alone (D: 3.0+-1.7% and H: 11.8+-3.9%) or with APP (D: 2.0+-1.5% and H: 61.3+-14.7%); VP26-GFP with gE (D: 21.8+-5.5%) or with LAMP2 (H: 4.6+-1.6%); and VP26-GFP with both APP and gE (D: 72.8+-6.7%), or both APP and LAMP2 (H: 22.3+-8.7%). Experiments were performed in triplicate, and 2085 particles in 11 cells were counted in D and 1592 particles in 9 cells in H. See Figure S5 for triple label of gD instead of gE, with APP and VP26-GFP.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
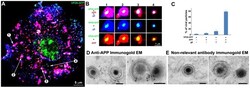
- Experimental details
- Figure 5 Confocal and immuno-gold electron microscopy demonstrate co-localization of both viral (gE) and cellular (APP) membrane proteins with VP26-GFP particles. (A) Example of a 0.8 um optical section by confocal imaging of a cell infected with VP26-GFP HSV1 (green), fixed at 8.5 hr p.i., and stained for cellular APP (red) and viral glycoprotein, gE (blue). (B) Galleries of particles showing the co-localization of VP26-GFP with gE and APP. (C) Histogram showing the percentage of VP26-GFP particles in each category. VP26-GFP alone (3.2+-2.0%), with APP (5.9+-2.4%), with gE (12.4+-5.6%) and with both APP and gE (78.4+-5.7%). 569 particles in 5 cells were counted. (D) Thin section immunogold electron microscopy of HSV1 infected cells probed with anti-C-APP with protein-A linked 10 nm gold particles. Note single and multiple gold particles decorating membranes surrounding viral capsids in the cytoplasm. Bar = 100 nm. (E) Parallel sections from the same EM block treated with an irrelevant rabbit antibody of similar purity and dilution and probed with protein-A gold. Note the absence of gold labeling of viral particles. Also see Figure S5 for co-localization of VP26-GFP particles with APP and viral protein gD, and Figure S6 for additional immunogold electron micrographs.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
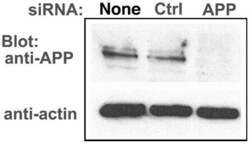
- Experimental details
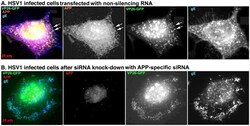
- Figure 6 APP knock-down by siRNA decreases APP protein >90% by Western blotting. HSV1 infected cells were transfected in parallel with either vehicle alone (None), non-silencing RNA (Ctrl) or siRNA against APP (APP). After 48 hr cells were scraped into lysis buffer, and loaded in parallel on a 10% gel for electrophoresis followed by transfer to nitrocellulose. The blot was divided in two horizontally, the top half probed for APP and the lower half for actin, a loading control. Non-silencing siRNA has little effect, while siRNA for APP decreases APP band intensity almost entirely, with no significant effect on actin.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 7 Knockdown of APP expression eliminates immuno-fluorescent staining of VP26-GFP particles by anti-APP antibodies. (A) Representative example of a cell treated with non-silencing control siRNA, infected with VP26-GFP HSV1 (green), fixed at 7 hr p.i. and stained for APP (red), gE (blue). APP staining is bright and diffuse, and co-localizes with VP26-GFP viral particles (arrows). (B) Representative example of a cell treated in parallel to the cell shown in (A) but with siRNA for APP. Note the absence of most APP staining, while gE staining of viral particles remains strong.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
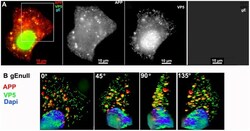
- Figure 3 APP co-localizes with gE virus. A representative example of a cell infected with gE virus stained for APP (red), gE (far red), and VP5 (green), the major capsid protein. (A) Low magnification wide-field image of the three channels, APP, VP5, and gE shown in color with merged channels (left), and then each individual channel, as labeled, of the same image. (B) Higher magnification of the boxed region shown in (A) and rendered in 3D. A Z-stack of 45 focal planes captured at 0.35 um intervals was deconvolved using iterative processing and rendered in 3D. In four angles of rotation, co-localization of green VP5 capsids and red APP membranes throughout the cytoplasm of a cell infected with gE virus is shown. See Movie S1 for a full rotation of the 3D stack shown in (B).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
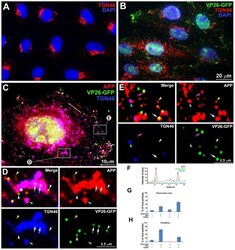
- Figure 10 APP and TGN46 do not co-localize with VP26-GFP labeled viral particles in the periphery of HSV1 infected cells: Evidence for specific APP-HSV1 interactions. (A) Uninfected cells stained for TGN46 (red) show compact tufts on one side of the DAPI-stained nuclei (blue). (B) Cells synchronously infected with VP26-GFP HSV1 (green), fixed and stained for TGN46 (red) and DAPI for nuclei (blue). (C) Higher magnification of an example of cell infected with VP26-GFP-HSV1 (green) fixed at 7 h p.i. and stained for TGN46 (blue) and APP (red). (D) Co-localization in the peri-nuclear region shown at high magnification of boxed region ""D"" in cell shown in (C). Arrows indicate co-localization of VP26-GFP particles with APP in a TGN46-stained compartment. White arrowhead indicates a VP26-GFP particle apparently on the surface of a TGN46-stained vesicle. Yellow and cyan arrowheads indicate examples of single anti-APP-stained and both APP and TGN46 stained particles, respectively. (E) Loss of co-localization at the periphery is shown at high magnification of boxed region E at the periphery of the cell shown in (C). Pink and white arrows indicate co-localization of VP26-GFP HSV1 with APP alone at the periphery and in the cytoplasm close to the periphery, respectively. Yellow and cyan arrowheads indicate examples of single APP and both APP and TGN46 labels, respectively. (F) Linescan intensity profile of a region in the intermediate cytoplasm as seen in (C) shows both coincident (arrow
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
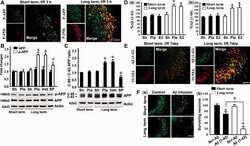
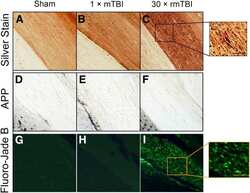
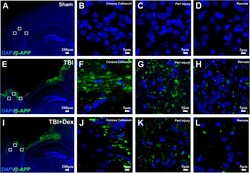
- Figure 4 Post-injury Dex treatment prevents axonal damage in the cortex. Representative images showed the beta-APP labeled damaged axon in the corpus callosum, peri-injury, and remote area of cortex of sham-injured, traumatic brain injury (TBI), and dexmedetomidine (Dex)-treated conditions. ( A-D ) Sham control mice showed no beta-APP staining of cortex. ( E-H ) After TBI, significant axonal damage is detected with beta-APP staining. ( J-L ) Dex treatment reduced the intensity of beta-APP staining.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
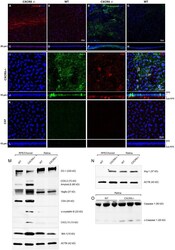
- Experimental details
- Figure 3 Immunofluorescent analysis of RPE/choroidal flat mounts and western blot analysis of RPE/Choroid and retinal material. Alpha-crystallin B (red) immunofluorescent staining in RPE/choroidal complexes. (A) Strong Alpha-crystallin B signals were detected in CXCR5 -/- animals; (B,D) noise levels of red fluorescence were detected in WT controls; z-stack depth analysis in (C) CXCR5 -/- flat mounts indicate sub-RPE localization of the signals in CXCR5 -/- RPE/choroidal complexes, forming a distinctive layer under the RPE cells. Marked amyloid beta (Abeta) staining (green) was detected in RPE/choroidal complexes in the (E) CXCR5 -/- sub-RPE localization of Abeta was identified by (F) z-stack depth analysis, no amyloid beta expression detected in (G) WT animals and (H) it's z-stacks. CXCR5 -/- mice also indicated an increased presence of IBA-1 (green) and CD4 (red) positive cells within the (I) RPE/choroidal complex; (J) z-stack analysis revealed the presence of IBA-1 positive signals on the surface of the RPE layer and sub-RPE localization of CD4 positive cells; (K,L) WT samples demonstrated minimal IBA-1 fluorescence and no CD4 positive cells. Nuclear counterstaining by DAPI (blue) in fluorescent images. Protein expression of (M) ZO-1, COX-2, CD4, Cryab, CXCL13, and VEGF-a; (N) Arg-1, expression CXCR5 -/- and age-matched WT control RPE/choroid and retina lysate. beta-Actin used as a loading control. (O) Cleavage of Caspase-1 in retina lysate of CXCR5 -/- and age-matched WT c
 Explore
Explore Validate
Validate Learn
Learn