Antibody data
- Antibody Data
- Antigen structure
- References [29]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [2]
- Other assay [13]
Submit
Validation data
Reference
Comment
Report error
- Product number
- P420B - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IL-1 beta Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Recombinant full-length protein
- Description
- P420B targets IL-1 beta in ELISA, IHC, Neu, and WB applications and shows reactivity with Human, mouse, and Non-human primate samples. The P420B immunogen is recombinant human IL-1 beta. P420B detects IL-1 beta which has a predicted molecular weight of approximately 31 kDa. This product is a Low Endotoxin formulation. This product has been tested for endotoxins by limulus amoebocyte lysate (LAL) assay and contains an endotoxin concentration of less than or equal to 10 endotoxin units per milligram (EU/mg).
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 1 mg
- Concentration
- 1.0 mg/mL
- Storage
- -20°C
Submitted references Placental Inflammasome mRNA Levels Differ by Mode of Delivery and Fetal Sex.
Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling.
IRF2 contributes to myocardial infarction via regulation of GSDMD induced pyroptosis.
Transcriptional and Histochemical Signatures of Bone Marrow Mononuclear Cell-Mediated Resolution of Synovitis.
Exenatide Attenuates Non-Alcoholic Steatohepatitis by Inhibiting the Pyroptosis Signaling Pathway.
Oxidation of Hemoglobin Drives a Proatherogenic Polarization of Macrophages in Human Atherosclerosis.
Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice.
Equine cervical remodeling during placentitis and the prepartum period: a transcriptomic approach.
Long-chain saturated fatty acids in breast milk are associated with the pathogenesis of atopic dermatitis via induction of inflammatory ILC3s.
The lack of functional DNMT2/TRDMT1 gene modulates cancer cell responses during drug-induced senescence.
Sterile inflammation drives multiple programmed cell death pathways in the gut.
Positive Allosteric Modulation of Alpha7 Nicotinic Acetylcholine Receptors Transiently Improves Memory but Aggravates Inflammation in LPS-Treated Mice.
p31-43 Gliadin Peptide Forms Oligomers and Induces NLRP3 Inflammasome/Caspase 1- Dependent Mucosal Damage in Small Intestine.
Dysregulated expression of antioxidant enzymes in polyethylene particle-induced periprosthetic inflammation and osteolysis.
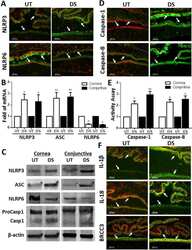
Desiccating stress worsens alkali burn injury by magnifying caspase-8-induced imbalance of NLRP3 and NLRP6.
Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye.
Spinal astrocytic activation contributes to mechanical allodynia in a mouse model of type 2 diabetes.
Distinct expression of interleukin-1α, interleukin-1β, and interleukin-1 receptor antagonist in testicular tissues and cells from human biopsies with normal and abnormal histology.
Cross-reactivity of anti-human, anti-porcine and anti-bovine cytokine antibodies with cetacean tissues.
Perfusion with lipopolysaccharide differently affects the secretion of interleukin-1 beta and interleukin-1 receptor antagonist by term and preterm human placentae.
Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection.
Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types.
Intrahepatic expression of interleukin-1beta and tumor necrosis factor-alpha in chronic hepatitis C.
1,25-Dihydroxyvitamin D3 and its analogues inhibit acute myelogenous leukemia progenitor proliferation by suppressing interleukin-1beta production.
Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms.
Activation of nuclear factor-kappa B and gene expression in human endothelial cells by the common haptens nickel and cobalt.
Tenidap-modulated proinflammatory cytokine activation of a monocyte cell line.
Induction of IL-3 and granulocyte-macrophage colony-stimulating factor by substance P in bone marrow cells is partially mediated through the release of IL-1 and IL-6.
Synergistic increases in IL-1 synthesis by the human monocytic cell line THP-1 treated with PAF and endotoxin.
Arthurs AL, Smith MD, Hintural MD, Breen J, McCullough D, Thornton FI, Leemaqz SY, Dekker GA, Jankovic-Karasoulos T, Roberts CT
Frontiers in immunology 2022;13:807750
Frontiers in immunology 2022;13:807750
Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling.
Kumar P, Lim A, Hazirah SN, Chua CJH, Ngoh A, Poh SL, Yeo TH, Lim J, Ling S, Sutamam NB, Petretto E, Low DCY, Zeng L, Tan EK, Arkachaisri T, Yeo JG, Ginhoux F, Chan D, Albani S
Nature neuroscience 2022 Jul;25(7):956-966
Nature neuroscience 2022 Jul;25(7):956-966
IRF2 contributes to myocardial infarction via regulation of GSDMD induced pyroptosis.
Li Y, Wang Y, Guo H, Wu Q, Hu Y
Molecular medicine reports 2022 Feb;25(2)
Molecular medicine reports 2022 Feb;25(2)
Transcriptional and Histochemical Signatures of Bone Marrow Mononuclear Cell-Mediated Resolution of Synovitis.
Menarim BC, El-Sheikh Ali H, Loux SC, Scoggin KE, Kalbfleisch TS, MacLeod JN, Dahlgren LA
Frontiers in immunology 2021;12:734322
Frontiers in immunology 2021;12:734322
Exenatide Attenuates Non-Alcoholic Steatohepatitis by Inhibiting the Pyroptosis Signaling Pathway.
Liu Y, Wang DW, Wang D, Duan BH, Kuang HY
Frontiers in endocrinology 2021;12:663039
Frontiers in endocrinology 2021;12:663039
Oxidation of Hemoglobin Drives a Proatherogenic Polarization of Macrophages in Human Atherosclerosis.
Potor L, Hendrik Z, Patsalos A, Katona É, Méhes G, Póliska S, Csősz É, Kalló G, Komáromi I, Combi Z, Posta N, Sikura KÉ, Pethő D, Oros M, Vereb G, Tóth C, Gergely P, Nagy L, Balla G, Balla J
Antioxidants & redox signaling 2021 Oct 20;35(12):917-950
Antioxidants & redox signaling 2021 Oct 20;35(12):917-950
Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice.
Reis-Mendes A, Padrão AI, Duarte JA, Gonçalves-Monteiro S, Duarte-Araújo M, Remião F, Carvalho F, Sousa E, Bastos ML, Costa VM
Biomolecules 2021 Nov 19;11(11)
Biomolecules 2021 Nov 19;11(11)
Equine cervical remodeling during placentitis and the prepartum period: a transcriptomic approach.
El-Sheikh Ali H, Scoggin KE, Ruby R, Loynachan A, Boakari Y, Fernandes C, Dini P, Fedorka CE, Loux SC, Esteller-Vico A, Ball BA
Reproduction (Cambridge, England) 2021 May 5;161(6):603-621
Reproduction (Cambridge, England) 2021 May 5;161(6):603-621
Long-chain saturated fatty acids in breast milk are associated with the pathogenesis of atopic dermatitis via induction of inflammatory ILC3s.
Kong WS, Tsuyama N, Inoue H, Guo Y, Mokuda S, Nobukiyo A, Nakatani N, Yamaide F, Nakano T, Kohno Y, Ikeda K, Nakanishi Y, Ohno H, Arita M, Shimojo N, Kanno M
Scientific reports 2021 Jun 23;11(1):13109
Scientific reports 2021 Jun 23;11(1):13109
The lack of functional DNMT2/TRDMT1 gene modulates cancer cell responses during drug-induced senescence.
Bloniarz D, Adamczyk-Grochala J, Lewinska A, Wnuk M
Aging 2021 Jun 17;13(12):15833-15874
Aging 2021 Jun 17;13(12):15833-15874
Sterile inflammation drives multiple programmed cell death pathways in the gut.
Ruera CN, Miculán E, Pérez F, Ducca G, Carasi P, Chirdo FG
Journal of leukocyte biology 2021 Jan;109(1):211-221
Journal of leukocyte biology 2021 Jan;109(1):211-221
Positive Allosteric Modulation of Alpha7 Nicotinic Acetylcholine Receptors Transiently Improves Memory but Aggravates Inflammation in LPS-Treated Mice.
Lykhmus O, Kalashnyk O, Uspenska K, Skok M
Frontiers in aging neuroscience 2019;11:359
Frontiers in aging neuroscience 2019;11:359
p31-43 Gliadin Peptide Forms Oligomers and Induces NLRP3 Inflammasome/Caspase 1- Dependent Mucosal Damage in Small Intestine.
Gómez Castro MF, Miculán E, Herrera MG, Ruera C, Perez F, Prieto ED, Barrera E, Pantano S, Carasi P, Chirdo FG
Frontiers in immunology 2019;10:31
Frontiers in immunology 2019;10:31
Dysregulated expression of antioxidant enzymes in polyethylene particle-induced periprosthetic inflammation and osteolysis.
Peng KT, Tsai MH, Lee CW, Chiang YC, Chen PC, Chen CC, Chang CH, Shih HN, Chang PJ
PloS one 2018;13(8):e0202501
PloS one 2018;13(8):e0202501
Desiccating stress worsens alkali burn injury by magnifying caspase-8-induced imbalance of NLRP3 and NLRP6.
Hua X, Yuan X, Li Y, Chen H, Yuan J, Tanumiharjo S, Bian F, Su L, Hong Y, Liu Y, Chi W
The Journal of allergy and clinical immunology 2017 Oct;140(4):1172-1176.e3
The Journal of allergy and clinical immunology 2017 Oct;140(4):1172-1176.e3
Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye.
Chi W, Hua X, Chen X, Bian F, Yuan X, Zhang L, Wang X, Chen D, Deng R, Li Z, Liu Y, de Paiva CS, Pflugfelder SC, Li DQ
Journal of autoimmunity 2017 Jun;80:65-76
Journal of autoimmunity 2017 Jun;80:65-76
Spinal astrocytic activation contributes to mechanical allodynia in a mouse model of type 2 diabetes.
Liao YH, Zhang GH, Jia D, Wang P, Qian NS, He F, Zeng XT, He Y, Yang YL, Cao DY, Zhang Y, Wang DS, Tao KS, Gao CJ, Dou KF
Brain research 2011 Jan 12;1368:324-35
Brain research 2011 Jan 12;1368:324-35
Distinct expression of interleukin-1α, interleukin-1β, and interleukin-1 receptor antagonist in testicular tissues and cells from human biopsies with normal and abnormal histology.
Abu Elheija M, Dyomin V, Ganaiem M, Lunenfeld E, Vardy NS, Huleihel M
Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 2011 Apr;31(4):401-8
Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 2011 Apr;31(4):401-8
Cross-reactivity of anti-human, anti-porcine and anti-bovine cytokine antibodies with cetacean tissues.
Jaber JR, Pérez J, Zafra R, Herráez P, Rodríguez F, Arbelo M, de los Monteros AE, Fernández A
Journal of comparative pathology 2010 Jul;143(1):45-51
Journal of comparative pathology 2010 Jul;143(1):45-51
Perfusion with lipopolysaccharide differently affects the secretion of interleukin-1 beta and interleukin-1 receptor antagonist by term and preterm human placentae.
Holcberg G, Amash A, Sapir O, Sheiner E, Levy S, Myatt L, Huleihel M
Placenta 2008 Jul;29(7):593-601
Placenta 2008 Jul;29(7):593-601
Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection.
Miura Y, Misawa N, Kawano Y, Okada H, Inagaki Y, Yamamoto N, Ito M, Yagita H, Okumura K, Mizusawa H, Koyanagi Y
Proceedings of the National Academy of Sciences of the United States of America 2003 Mar 4;100(5):2777-82
Proceedings of the National Academy of Sciences of the United States of America 2003 Mar 4;100(5):2777-82
Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types.
Andjelkovic AV, Kerkovich D, Pachter JS
Journal of leukocyte biology 2000 Oct;68(4):545-52
Journal of leukocyte biology 2000 Oct;68(4):545-52
Intrahepatic expression of interleukin-1beta and tumor necrosis factor-alpha in chronic hepatitis C.
Dumoulin FL, Leifeld L, Honecker U, Sauerbruch T, Spengler U
The Journal of infectious diseases 1999 Nov;180(5):1704-8
The Journal of infectious diseases 1999 Nov;180(5):1704-8
1,25-Dihydroxyvitamin D3 and its analogues inhibit acute myelogenous leukemia progenitor proliferation by suppressing interleukin-1beta production.
Peleg S, Qiu H, Reddy S, Harris D, Van Q, Estey EH, Talpaz M, Estrov Z
The Journal of clinical investigation 1997 Oct 1;100(7):1716-24
The Journal of clinical investigation 1997 Oct 1;100(7):1716-24
Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms.
Waldburger KE, Hastings RC, Schaub RG, Goldman SJ, Leonard JP
The American journal of pathology 1996 Feb;148(2):375-82
The American journal of pathology 1996 Feb;148(2):375-82
Activation of nuclear factor-kappa B and gene expression in human endothelial cells by the common haptens nickel and cobalt.
Goebeler M, Roth J, Bröcker EB, Sorg C, Schulze-Osthoff K
Journal of immunology (Baltimore, Md. : 1950) 1995 Sep 1;155(5):2459-67
Journal of immunology (Baltimore, Md. : 1950) 1995 Sep 1;155(5):2459-67
Tenidap-modulated proinflammatory cytokine activation of a monocyte cell line.
Golding S, Emery P, Young SP
Journal of immunology (Baltimore, Md. : 1950) 1995 May 15;154(10):5384-90
Journal of immunology (Baltimore, Md. : 1950) 1995 May 15;154(10):5384-90
Induction of IL-3 and granulocyte-macrophage colony-stimulating factor by substance P in bone marrow cells is partially mediated through the release of IL-1 and IL-6.
Rameshwar P, Ganea D, Gascón P
Journal of immunology (Baltimore, Md. : 1950) 1994 Apr 15;152(8):4044-54
Journal of immunology (Baltimore, Md. : 1950) 1994 Apr 15;152(8):4044-54
Synergistic increases in IL-1 synthesis by the human monocytic cell line THP-1 treated with PAF and endotoxin.
Barthelson RA, Potter T, Valone FH
Cellular immunology 1990 Jan;125(1):142-50
Cellular immunology 1990 Jan;125(1):142-50
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
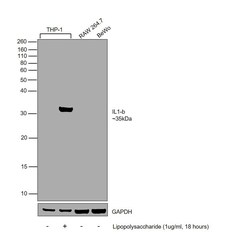
- Experimental details
- Western blot was performed using Anti-IL-1 beta Polyclonal Antibody (Product # P420B) and a 35 kDa band corresponding to Interleukin-1 beta was observed across in THP-1, upon treatment with Lipopolysaccharide. Whole cell extracts (30 µg lysate) of THP-1 (Lane 1), THP-1 (Lipopolysaccharide (1 µg/mL), 18 hours) (Lane 2), RAW 264.7 (Lane 3), BeWo (Lane 4) were electrophoresed using NuPAGE™ 12% Bis-Tris Protein Gel (Product # NP0342BOX). Resolved proteins were then transferred onto a Nitrocellulose membrane (Product # IB23001) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (1 µg/mL) and detected by chemiluminescence with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A27036, 1:4000) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
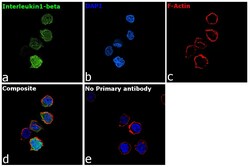
- Experimental details
- Immunofluorescence analysis of Interleukin-1 beta was performed using 70% confluent log phase THP-1 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with IL-1 beta Polyclonal Antibody (Product # P420B) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731, 1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
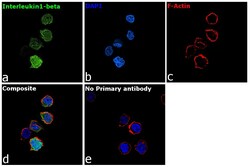
- Experimental details
- Immunofluorescence analysis of Interleukin-1 beta was performed using 70% confluent log phase THP-1 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with IL-1 beta Polyclonal Antibody (Product # P420B) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731, 1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
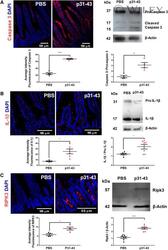
- 2 FIGURE Intragastric treatment with gliadin p31-43 peptide induces apoptosis, pyroptosis, and necroptosis . ( A ) Left: representative images of caspase-3 staining of small intestine of C57BL/6 mice 16 h after intragastric administration of PBS or 20 µg gliadin p31-43. DAPI staining for nuclei (blue), caspase-3 in red. Fluorescence intensity of caspase-3 staining in histological sections of small intestine 16 h after administration of p31-43 ( n = 5) and in controls ( n = 4). **** P < 0.0001. Student's unpaired t -test. Right: representative Western blot analysis of procaspase-3 and cleaved caspase-3 in small intestine 16 h after administration of p31-43 or PBS. Quantitative analysis of Western blot bands expressed as the ratio of cleaved caspase-3:procaspase-3 in p31-43 ( n = 4) or PBS ( n = 4)-treated mice. ** P < 0.01; Student's unpaired t -test. ( B ) Left: representative images of IL-1beta staining of small intestine of C57BL/6 mice 16 h after intragastric administration of PBS or 20 µg gliadin p31-43. DAPI staining for nuclei (blue), IL-1beta in red. Fluorescence intensity of IL-1beta staining in histological sections of small intestine 16 h after administration of p31-43 ( n = 4) and in controls ( n = 6). * P < 0.05; Student's unpaired t -test. Right: representative Western blot analysis of IL-1beta activation in small intestine 16 h after administration of p31-43 or PBS. Quantitative analysis expressed as the ratio of mature IL-1beta: pro-IL-1beta 16 h af
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
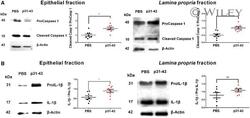
- 5 FIGURE Induction of pyroptosis in p31-43-treated mice . ( A ) Representative Western blot analysis and quantitative analysis of caspase-1 activation expressed as the ratio of cleaved caspase-1:procaspase-1 in epithelial and lamina propria fractions 16 h after administration of p31-43 ( n = 8) or PBS ( n = 8). ** P < 0.01; Student's unpaired t -test. ( B ) Representative Western blot analysis and quantitative analysis of IL-1beta activation expressed as the ratio of mature IL-1beta:pro-IL-1beta in epithelial and lamina propria fractions 16 h after administration of p31-43 ( n = 8) or PBS ( n = 8). * P < 0.05; Student's unpaired t -test
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
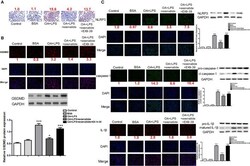
- Figure 1 Exenatide attenuated NASH by inhibiting the pyroptosis signaling pathway in vitro . (A) A representative Oil Red O staining image showed that exenatide attenuated NASH. The numbers above each picture (shown in red) indicated the relative staining intensity as quantified by ImageJ. (B) Representative immunofluorescence images of GSDMD and GSDMD protein expression levels measured by western blotting. (C) Representative immunofluorescence images of NLRP3, caspase-1, and IL-1beta. NLRP3, caspase-1, and IL-1beta. Protein expression levels were measured by western blotting. For panel (B, C) , the numbers below the images (shown in red) indicated the relative fluorescence intensity as quantified by ImageJ. Two subcultures were used for each western blot markers and the experiment was repeated 2-3 times. *p < 0.1; ***p < 0.001. Data are expressed as the mean +- SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
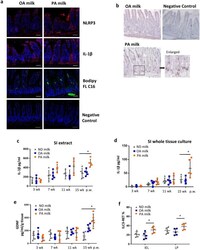
- Figure 6 PA-milk diet induced inflammation in the gut and skin. ( a ) Representative immunofluorescence staining for NLRP3 (red), IL-1beta (red) and Bodipy FL C16 (green) in the small intestine of mice at 15 weeks post-weaning (p.w.). Nuclei were stained with DAPI (blue). Scale bars, 100 um. ( b ) Oil red O lipid staining of the small intestines of mice at 15 p.w. ELISA quantitation of IL-1beta (pg/ml) in ( c ) small intestine (SI) extracts and ( d ) whole tissue culture taken from mice at indicated p.w. (n = 5 mice/group). SI extracts were obtained by lysis treatment and sonication. For whole tissue culture, small intestine was cut into small pieces and cultured on 24-well tissue dish for 18 h before the collection of supernatant for IL-1beta quantitation. ( e ) ELISA quantitation of GDNF in small intestine extracts at indicated p.w. ( f ) Percentages of ILC3-RET expression in IEL and LP of mice at 15 weeks p.w. (n = 8 mice/group). * P < 0.05, ** P < 0.01, one-way ANOVA. Data are analyzed with Prism8 (version 8.4.3, https://www.graphpad.com/scientific-software/prism ), and ImageJ (version 1.53, https://imagej.nih.gov/ij/index.html ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
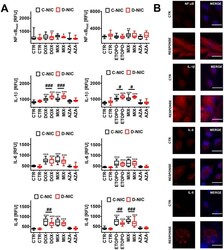
- Experimental details
- Figure 7 DNMT2/TRDMT1 gene knockout-mediated senescence-associated secretory phenotype (SASP) ( A , B ) in U-251 MG glioblastoma cells treated with DOX or ETOPO. ( A ) The levels of NF-kappaB, IL-1beta, IL-6 and IL-8 are expressed as relative fluorescence units (RFU). Box and whisker plots are shown, n = 3, *** p < 0.001, ** p < 0.01, * p < 0.05 compared to CTR (ANOVA and Dunnett's a posteriori test), ### p < 0.001, ## p < 0.01, # p < 0.05 compared to C-NIC cells at the same culture conditions (ANOVA and Tukey's a posteriori test). ( B ) NF-kappaB, IL-1beta, IL-6 and IL-8 immunostaining (red). Representative microphotographs are shown, objective 20x, nucleus staining (blue), RESPONSE, representative DOX or ETOPO treatment. CTR, control conditions; DOX, doxorubicin treatment; ETOPO, etoposide treatment; AZA, azacytidine treatment; MIX, azacytidine post-treatment; C-NIC, control cells with unmodified levels of DNMT2/TRDMT1 containing control plasmid; D-NIC, cells with DNMT2/TRDMT1 gene knockout containing dedicated DNMT2 double nickase plasmid.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
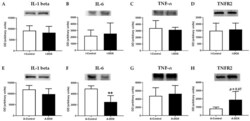
- Experimental details
- Figure 10 ( A , E ) Interleukin-1 beta (IL-1 beta) (35 kDa), ( B , F ) interleukin-6 (IL-6) (24 kDa), ( C , G ) tumor necrosis factor- alpha (TNF-alpha) (25 kDa), ( D , H ) type 2 TNF receptor (TNFR2) (75 kDa) levels in cardiac tissue evaluated by Western blotting, in infant DOX-treated (I-DOX) and control mice (I-Control), and adult mice exposed to a cumulative dose of 18.0 mg/kg DOX (A-DOX) and respective controls (A-Control). Values are expressed as mean +- SD and were obtained from 6 (infant) and 6-7 (adult) animals from each treatment group. Statistical comparisons were made using Student's t -test: ** p < 0.01, DOX vs. control. OD: optic density. Protein loading was confirmed by the Ponceau S staining ( Figure S3 ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
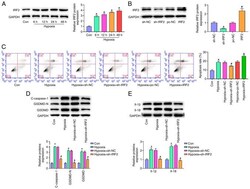
- Experimental details
- Figure 4. IRF2 silencing attenuates GSDMD-induced pyroptosis in vitro . (A) IRF-2 expression in H9c2 cells stimulated by hypoxia (6, 12, 24 and 48 h) was detected by reverse transcription-quantitative PCR. *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- FIG. 13. Transcriptomic analysis reveals that ferryl hemoglobin polarizes macrophages into a proinflammatory-like phenotype. (A, B) RNA-seq transcriptomic analysis was performed on human macrophages treated with ferrylHb (10 mu M ) for 8 h ( n = 4/group). (A) Heatmap of the 50 most-changed DE genes is shown in log 10 (FC). (B) M1 and M2 signatures are shown as a clustered heatmap in log 10 (FC). (C, D) Relative expression of (C) IL-1beta, TNF-alpha, CXCL8, (D) CD209, and IL-27RA were analyzed by real-time qPCR ( n = 3). (E) Protein expression of HO-1, H-ferritin, Pro-IL-1beta, IL-1beta, TNF-alpha and GAPDH was determined by Western blots in macrophages exposed to ferrylHb (10 mu M , 8-h ferrylHb treatment). ** p < 0.01; *** p < 0.001. DE, differentially expressed; FC, fold change. Color images are available online.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
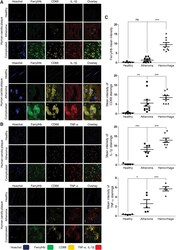
- Experimental details
- FIG. 6. Ferryl hemoglobin-positive macrophages in carotid artery are positive for IL-1beta and TNF-alpha. Cross sections of a healthy carotid artery and atheromatous and hemorrhagic transformed lesions were shown. (A) Images demonstrated macrophages positive for both CD68 and IL-1beta in the atheromatous plaque and positive for ferrylHb, CD68, and IL-1beta in the hemorrhagic transformed lesions. Sections were stained with Hoechst 33258 for DNA ( blue ), an anti-ferrylHb antibody with Alexa Flour 488 secondary antibody for ferrylHb ( green ), an anti-CD68 antibody with Alexa Flour 568 secondary antibody for CD68 ( yellow ), and an anti-IL-1beta antibody with Alexa Flour 647 secondary antibody for IL-1beta ( red ). (B) Images demonstrated macrophages positive for both CD68 and TNF-alpha in the atheromatous plaque and positive for ferrylHb, CD68, and TNF-alpha in the hemorrhagic transformed lesions. Sections were stained with Hoechst 33258 for DNA ( blue ), an anti-ferrylHb antibody with Alexa Flour 488 secondary antibody for ferrylHb ( green ), an anti-CD68 antibody with Alexa Flour 568 secondary antibody for CD68 ( yellow ), and an anti-TNF-alpha antibody with Alexa Flour 647 secondary antibody for TNF-alpha ( red ). Images were taken using Leica TCS SP8 gated STED-CW nanoscopy. Images were deconvolved using Huygens Professional software. Representative image, N = 5. (C) Fluorescence intensity for ferrylHb ( n = 17), CD68 ( n = 20), IL-1beta ( n = 19), and TNF-alpha ( n = 15)
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
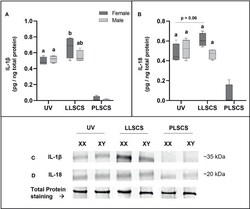
- The mRNA expression of Interleukin 1beta (IL-1beta) (A, C) and Interleukin 18 (IL-18) (B, D) protein in placentae from female- and male-bearing pregnancies classed by delivery mode. For (A, B) , Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (C, D) show representative images of staining with an IL-1beta antibody (molecular weight ~35 kDa) and an IL-18 antibody (molecular weight ~20 kDa), respectively. Below this is a representative image of stain-free Total Protein staining as a control. (Number of samples: unassisted vaginal (UV) = 6/fetal sex; labouring lower segment Caesarean section (LLSCS) = 4/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 2/fetal sex).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
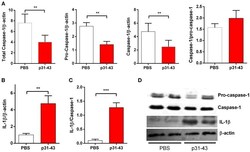
- Figure 5 Oral administration of p31-43 induces caspase 1 activation and production of mature IL-1beta. (A) Western blot analysis of mature IL-1beta and (B) caspase-1 and pro-caspase-1 quantification in whole small intestinal tissue from p31-43 or vehicle-fed mice 16 h after treatment. (C) IL-1beta/caspase 1 ratio of data shown in (A,B) . (D) Representative results of Western blots are shown. Results shown are means +- 1 SEM for 5 mice and are representative of two experiments. ** p < 0.01, *** p < 0.001, Student's t -test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
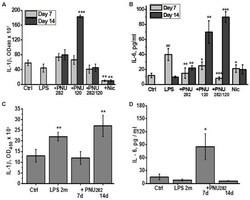
- Figure 2 Effects of PNU282, PNU120, their combination, or nicotine on IL-1beta (A,C) or IL-6 (B,D) levels in the blood of mice on days 7 and 14 after LPS injection (A,B) or on days 7 and 14, 2 months after LPS injection (C,D) . Each column corresponds to Mean +- SD, n = 5. (A,B) * p < 0.05; ** p < 0.005; *** p < 0.0005 compared to LPS; ## p < 0.005 compared to Ctrl. (C,D) * p < 0.05; ** p < 0.005; compared to Ctrl.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA