Antibody data
- Antibody Data
- Antigen structure
- References [52]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [30]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 11-0118-41 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD11b Monoclonal Antibody (ICRF44), FITC, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The ICRF44 monoclonal antibody reacts with human CD11b, a 165 kDa adhesion molecule. CD11b associated with integrin beta2 (CD18) is expressed on the surface of monocytes, granulocytes, activated lymphocytes and a subset of NK cells. CD11b is a receptor for intercellular adhesion molecule family members CD54, CD102 and CD50 as well as for iC3b. These adhesions are crucial in cell-cell and cell-matrix interactions. Applications Reported: This ICRF44 antibody has been reported for use in flow cytometric analysis. Applications Tested: This ICRF44 antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (0.5 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 488 nm; Emission: 520 nm; Laser: Blue Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Conjugate
- Green dye
- Isotype
- IgG
- Antibody clone number
- ICRF44
- Vial size
- 25 Tests
- Concentration
- 5 µL/Test
- Storage
- 4° C, store in dark, DO NOT FREEZE!
Submitted references SARS-CoV-2 Delta (B.1.617.2) variant replicates and induces syncytia formation in human induced pluripotent stem cell-derived macrophages.
LAL deficiency induced myeloid-derived suppressor cells as targets and biomarkers for lung cancer.
Heliangin acts as a covalent ligand of RPS2 that disrupts pre-rRNA metabolic processes in NPM1-mutated acute myeloid leukemia.
Anti-DEspR antibody treatment improves survival and reduces neurologic deficits in a hypertensive, spontaneous intracerebral hemorrhage (hsICH) rat model.
Glucocorticoid activation of anti-inflammatory macrophages protects against insulin resistance.
Prognostic Lnc-S100B-2 Affects Cell Apoptosis and Microenvironment of Colorectal Cancer through MLLT10 Signaling.
The Transcription of ZIP9 Is Associated With the Macrophage Polarization and the Pathogenesis of Hepatocellular Carcinoma.
Methylation of SPRED1: A New Target in Acute Myeloid Leukemia.
Post-amputation reactive oxygen species production is necessary for axolotls limb regeneration.
Chemical optimization of siRNA for safe and efficient silencing of placental sFLT1.
REDD1 promotes obesity-induced metabolic dysfunction via atypical NF-κB activation.
Endothelial dysfunction markers and immune response indices in cosmonauts' blood after long-duration space flights.
Stem cell architecture drives myelodysplastic syndrome progression and predicts response to venetoclax-based therapy.
A single-cell atlas of the normal and malformed human brain vasculature.
miR-204-containing exosomes ameliorate GVHD-associated dry eye disease.
Associations between Hypertriglyceridemia and Circulating Neutrophil Subpopulation in Patients with Dyslipidemia.
Different Induction of PD-L1 (CD274) and PD-1 (CD279) Expression in THP-1-Differentiated Types 1 and 2 Macrophages.
G-MDSCs promote aging-related cardiac fibrosis by activating myofibroblasts and preventing senescence.
Heterogeneous disease-propagating stem cells in juvenile myelomonocytic leukemia.
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Connecting METTL3 and intratumoural CD33(+) MDSCs in predicting clinical outcome in cervical cancer.
Single-Cell Analyses Reveal Megakaryocyte-Biased Hematopoiesis in Myelofibrosis and Identify Mutant Clone-Specific Targets.
Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation.
Ubiquitin-specific peptidase 3 induces TPA-mediated leukemia cell differentiation via regulating H2AK119ub.
An altered response in macrophage phenotype following damage in aged human skeletal muscle: implications for skeletal muscle repair.
Tumor-Contacted Neutrophils Promote Metastasis by a CD90-TIMP-1 Juxtacrine-Paracrine Loop.
Deacetylase activity-independent transcriptional activation by HDAC2 during TPA-induced HL-60 cell differentiation.
Microglia innately develop within cerebral organoids.
Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis.
NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification.
AURKA Suppresses Leukemic THP-1 Cell Differentiation through Inhibition of the KDM6B Pathway.
Accumulation of T-helper 22 cells, interleukin-22 and myeloid-derived suppressor cells promotes gastric cancer progression in elderly patients.
n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils.
Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner.
Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection.
The Correlation of CD206, CD209, and Disease Severity in Behçet's Disease with Arthritis.
Differential regulation of innate immune cytokine production through pharmacological activation of Nuclear Factor-Erythroid-2-Related Factor 2 (NRF2) in burn patient immune cells and monocytes.
The prognostic significance of CD11b(+)CX3CR1(+) monocytes in patients with newly diagnosed diffuse large B-cell lymphoma.
Dichotomous Expression of TNF Superfamily Ligands on Antigen-Presenting Cells Controls Post-priming Anti-viral CD4(+) T Cell Immunity.
TRIB3 Promotes APL Progression through Stabilization of the Oncoprotein PML-RARα and Inhibition of p53-Mediated Senescence.
CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity.
Pharmacological Inhibition of the Histone Lysine Demethylase KDM1A Suppresses the Growth of Multiple Acute Myeloid Leukemia Subtypes.
Induction of autophagy is a key component of all-trans-retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacologic modulation.
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma.
Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells.
Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis.
Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity.
Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells.
Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages.
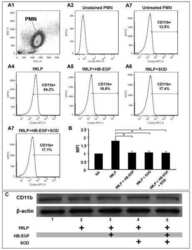
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) preserves gut barrier function by blocking neutrophil-endothelial cell adhesion after hemorrhagic shock and resuscitation in mice.
Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties.
Thaweerattanasinp T, Wanitchang A, Saenboonrueng J, Srisutthisamphan K, Wanasen N, Sungsuwan S, Jongkaewwattana A, Chailangkarn T
PeerJ 2023;11:e14918
PeerJ 2023;11:e14918
LAL deficiency induced myeloid-derived suppressor cells as targets and biomarkers for lung cancer.
Zhao T, Liu S, Hanna NH, Jalal S, Ding X, Wan J, Yan C, Du H
Journal for immunotherapy of cancer 2023 Mar;11(3)
Journal for immunotherapy of cancer 2023 Mar;11(3)
Heliangin acts as a covalent ligand of RPS2 that disrupts pre-rRNA metabolic processes in NPM1-mutated acute myeloid leukemia.
Feng Y, Han Y, Hu A, Qu Y, Hu Y, Wu H, Wang X, He L
Acta pharmaceutica Sinica. B 2023 Feb;13(2):598-617
Acta pharmaceutica Sinica. B 2023 Feb;13(2):598-617
Anti-DEspR antibody treatment improves survival and reduces neurologic deficits in a hypertensive, spontaneous intracerebral hemorrhage (hsICH) rat model.
Herrera VLM, Gromisch CM, Decano JL, Pasion KA, Tan GLA, Hua N, Takahashi CE, Greer DM, Ruiz-Opazo N
Scientific reports 2023 Feb 15;13(1):2703
Scientific reports 2023 Feb 15;13(1):2703
Glucocorticoid activation of anti-inflammatory macrophages protects against insulin resistance.
Caratti G, Stifel U, Caratti B, Jamil AJM, Chung KJ, Kiehntopf M, Gräler MH, Blüher M, Rauch A, Tuckermann JP
Nature communications 2023 Apr 20;14(1):2271
Nature communications 2023 Apr 20;14(1):2271
Prognostic Lnc-S100B-2 Affects Cell Apoptosis and Microenvironment of Colorectal Cancer through MLLT10 Signaling.
Yi J, Peng F, Zhao J, Gong X
Journal of oncology 2022;2022:3565118
Journal of oncology 2022;2022:3565118
The Transcription of ZIP9 Is Associated With the Macrophage Polarization and the Pathogenesis of Hepatocellular Carcinoma.
Gou Y, Yang D, Tian T, Zhu X, Zhang R, Ren J, Tu D, Luo Y, Miao Y, Zhao H, Wang Y, Wei B
Frontiers in immunology 2022;13:725595
Frontiers in immunology 2022;13:725595
Methylation of SPRED1: A New Target in Acute Myeloid Leukemia.
Su N, Wang Y, Lu X, Xu W, Wang H, Mo W, Pang H, Tang R, Li S, Yan X, Li Y, Zhang R
Frontiers in oncology 2022;12:854192
Frontiers in oncology 2022;12:854192
Post-amputation reactive oxygen species production is necessary for axolotls limb regeneration.
Carbonell-M B, Zapata Cardona J, Delgado JP
Frontiers in cell and developmental biology 2022;10:921520
Frontiers in cell and developmental biology 2022;10:921520
Chemical optimization of siRNA for safe and efficient silencing of placental sFLT1.
Davis SM, Hariharan VN, Lo A, Turanov AA, Echeverria D, Sousa J, McHugh N, Biscans A, Alterman JF, Karumanchi SA, Moore MJ, Khvorova A
Molecular therapy. Nucleic acids 2022 Sep 13;29:135-149
Molecular therapy. Nucleic acids 2022 Sep 13;29:135-149
REDD1 promotes obesity-induced metabolic dysfunction via atypical NF-κB activation.
Lee DK, Kim T, Byeon J, Park M, Kim S, Kim J, Choi S, Lee G, Park C, Lee KW, Kwon YJ, Lee JH, Kwon YG, Kim YM
Nature communications 2022 Oct 22;13(1):6303
Nature communications 2022 Oct 22;13(1):6303
Endothelial dysfunction markers and immune response indices in cosmonauts' blood after long-duration space flights.
Kuzichkin DS, Nichiporuk IA, Zhuravleva OA, Markin AA, Rykova MP, Zhuravleva TV, Sadova AA, Kutko OV, Shmarov VA, Ponomarev SA
NPJ microgravity 2022 Nov 2;8(1):46
NPJ microgravity 2022 Nov 2;8(1):46
Stem cell architecture drives myelodysplastic syndrome progression and predicts response to venetoclax-based therapy.
Ganan-Gomez I, Yang H, Ma F, Montalban-Bravo G, Thongon N, Marchica V, Richard-Carpentier G, Chien K, Manyam G, Wang F, Alfonso A, Chen S, Class C, Kanagal-Shamanna R, Ingram JP, Ogoti Y, Rose A, Loghavi S, Lockyer P, Cambo B, Muftuoglu M, Schneider S, Adema V, McLellan M, Garza J, Marchesini M, Giuliani N, Pellegrini M, Wang J, Walker J, Li Z, Takahashi K, Leverson JD, Bueso-Ramos C, Andreeff M, Clise-Dwyer K, Garcia-Manero G, Colla S
Nature medicine 2022 Mar;28(3):557-567
Nature medicine 2022 Mar;28(3):557-567
A single-cell atlas of the normal and malformed human brain vasculature.
Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, Chen LQ, Wu D, Catapano JS, Raygor K, Narsinh K, Kim H, Weinsheimer S, Cooke DL, Walcott BP, Lawton MT, Gupta N, Zlokovic BV, Chang EF, Abla AA, Lim DA, Nowakowski TJ
Science (New York, N.Y.) 2022 Mar 4;375(6584):eabi7377
Science (New York, N.Y.) 2022 Mar 4;375(6584):eabi7377
miR-204-containing exosomes ameliorate GVHD-associated dry eye disease.
Zhou T, He C, Lai P, Yang Z, Liu Y, Xu H, Lin X, Ni B, Ju R, Yi W, Liang L, Pei D, Egwuagu CE, Liu X
Science advances 2022 Jan 14;8(2):eabj9617
Science advances 2022 Jan 14;8(2):eabj9617
Associations between Hypertriglyceridemia and Circulating Neutrophil Subpopulation in Patients with Dyslipidemia.
Genkel V, Dolgushin I, Baturina I, Savochkina A, Kuznetsova A, Pykhova L, Shaposhnik I
International journal of inflammation 2021;2021:6695468
International journal of inflammation 2021;2021:6695468
Different Induction of PD-L1 (CD274) and PD-1 (CD279) Expression in THP-1-Differentiated Types 1 and 2 Macrophages.
Lai CY, Tseng PC, Chen CL, Satria RD, Wang YT, Lin CF
Journal of inflammation research 2021;14:5241-5249
Journal of inflammation research 2021;14:5241-5249
G-MDSCs promote aging-related cardiac fibrosis by activating myofibroblasts and preventing senescence.
Sun SN, Ni SH, Li Y, Liu X, Deng JP, Chen ZX, Li H, Feng WJ, Huang YS, Li DN, Xian SX, Yang ZQ, Wang LJ, Lu L
Cell death & disease 2021 Jun 8;12(6):594
Cell death & disease 2021 Jun 8;12(6):594
Heterogeneous disease-propagating stem cells in juvenile myelomonocytic leukemia.
Louka E, Povinelli B, Rodriguez-Meira A, Buck G, Wen WX, Wang G, Sousos N, Ashley N, Hamblin A, Booth CAG, Roy A, Elliott N, Iskander D, de la Fuente J, Fordham N, O'Byrne S, Inglott S, Norfo R, Salio M, Thongjuea S, Rao A, Roberts I, Mead AJ
The Journal of experimental medicine 2021 Feb 1;218(2)
The Journal of experimental medicine 2021 Feb 1;218(2)
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Grasedieck S, Ruess C, Krowiorz K, Lux S, Pochert N, Schwarzer A, Klusmann JH, Jongen-Lavrencic M, Herold T, Bullinger L, Pollack JR, Rouhi A, Kuchenbauer F
Haematologica 2020 Sep 1;105(9):e448-453
Haematologica 2020 Sep 1;105(9):e448-453
Connecting METTL3 and intratumoural CD33(+) MDSCs in predicting clinical outcome in cervical cancer.
Ni HH, Zhang L, Huang H, Dai SQ, Li J
Journal of translational medicine 2020 Oct 15;18(1):393
Journal of translational medicine 2020 Oct 15;18(1):393
Single-Cell Analyses Reveal Megakaryocyte-Biased Hematopoiesis in Myelofibrosis and Identify Mutant Clone-Specific Targets.
Psaila B, Wang G, Rodriguez-Meira A, Li R, Heuston EF, Murphy L, Yee D, Hitchcock IS, Sousos N, O'Sullivan J, Anderson S, Senis YA, Weinberg OK, Calicchio ML, NIH Intramural Sequencing Center, Iskander D, Royston D, Milojkovic D, Roberts I, Bodine DM, Thongjuea S, Mead AJ
Molecular cell 2020 May 7;78(3):477-492.e8
Molecular cell 2020 May 7;78(3):477-492.e8
Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation.
Zhang CX, Huang DJ, Baloche V, Zhang L, Xu JX, Li BW, Zhao XR, He J, Mai HQ, Chen QY, Zhang XS, Busson P, Cui J, Li J
Oncogenesis 2020 Jul 6;9(7):65
Oncogenesis 2020 Jul 6;9(7):65
Ubiquitin-specific peptidase 3 induces TPA-mediated leukemia cell differentiation via regulating H2AK119ub.
Chae YC, Jung H, Kim JY, Lee DH, Seo SB
Animal cells and systems 2019;23(5):311-317
Animal cells and systems 2019;23(5):311-317
An altered response in macrophage phenotype following damage in aged human skeletal muscle: implications for skeletal muscle repair.
Sorensen JR, Kaluhiokalani JP, Hafen PS, Deyhle MR, Parcell AC, Hyldahl RD
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019 Sep;33(9):10353-10368
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019 Sep;33(9):10353-10368
Tumor-Contacted Neutrophils Promote Metastasis by a CD90-TIMP-1 Juxtacrine-Paracrine Loop.
Wang Y, Chen J, Yang L, Li J, Wu W, Huang M, Lin L, Su S
Clinical cancer research : an official journal of the American Association for Cancer Research 2019 Mar 15;25(6):1957-1969
Clinical cancer research : an official journal of the American Association for Cancer Research 2019 Mar 15;25(6):1957-1969
Deacetylase activity-independent transcriptional activation by HDAC2 during TPA-induced HL-60 cell differentiation.
Jung H, Kim JY, Kim KB, Chae YC, Hahn Y, Kim JW, Seo SB
PloS one 2018;13(8):e0202935
PloS one 2018;13(8):e0202935
Microglia innately develop within cerebral organoids.
Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ
Nature communications 2018 Oct 9;9(1):4167
Nature communications 2018 Oct 9;9(1):4167
Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis.
Cambré I, Gaublomme D, Burssens A, Jacques P, Schryvers N, De Muynck A, Meuris L, Lambrecht S, Carter S, de Bleser P, Saeys Y, Van Hoorebeke L, Kollias G, Mack M, Simoens P, Lories R, Callewaert N, Schett G, Elewaut D
Nature communications 2018 Nov 5;9(1):4613
Nature communications 2018 Nov 5;9(1):4613
NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification.
Barruet E, Morales BM, Cain CJ, Ton AN, Wentworth KL, Chan TV, Moody TA, Haks MC, Ottenhoff TH, Hellman J, Nakamura MC, Hsiao EC
JCI insight 2018 Nov 15;3(22)
JCI insight 2018 Nov 15;3(22)
AURKA Suppresses Leukemic THP-1 Cell Differentiation through Inhibition of the KDM6B Pathway.
Park JW, Cho H, Oh H, Kim JY, Seo SB
Molecules and cells 2018 May 31;41(5):444-453
Molecules and cells 2018 May 31;41(5):444-453
Accumulation of T-helper 22 cells, interleukin-22 and myeloid-derived suppressor cells promotes gastric cancer progression in elderly patients.
Chen X, Wang Y, Wang J, Wen J, Jia X, Wang X, Zhang H
Oncology letters 2018 Jul;16(1):253-261
Oncology letters 2018 Jul;16(1):253-261
n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils.
Wu B, Wang L, Jiang L, Dong L, Xu F, Lu Y, Jin J, Wang Z, Liang G, Shan X
Molecular medicine reports 2018 Jan;17(1):179-185
Molecular medicine reports 2018 Jan;17(1):179-185
Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner.
Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S
Cell 2018 Jan 25;172(3):500-516.e16
Cell 2018 Jan 25;172(3):500-516.e16
Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection.
Hong D, Ding J, Li O, He Q, Ke M, Zhu M, Liu L, Ou WB, He Y, Wu Y
Stem cell research & therapy 2018 Feb 26;9(1):49
Stem cell research & therapy 2018 Feb 26;9(1):49
The Correlation of CD206, CD209, and Disease Severity in Behçet's Disease with Arthritis.
Choi B, Suh CH, Kim HA, Sayeed HM, Sohn S
Mediators of inflammation 2017;2017:7539529
Mediators of inflammation 2017;2017:7539529
Differential regulation of innate immune cytokine production through pharmacological activation of Nuclear Factor-Erythroid-2-Related Factor 2 (NRF2) in burn patient immune cells and monocytes.
Eitas TK, Stepp WH, Sjeklocha L, Long CV, Riley C, Callahan J, Sanchez Y, Gough P, Knowlin L, van Duin D, Ortiz-Pujols S, Jones SW, Maile R, Hong Z, Berger S, Cairns BA
PloS one 2017;12(9):e0184164
PloS one 2017;12(9):e0184164
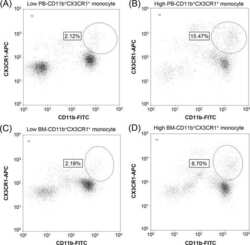
The prognostic significance of CD11b(+)CX3CR1(+) monocytes in patients with newly diagnosed diffuse large B-cell lymphoma.
Yhim HY, Kim JA, Ko SH, Park Y, Yim E, Kim HS, Kwak JY
Oncotarget 2017 Nov 3;8(54):92289-92299
Oncotarget 2017 Nov 3;8(54):92289-92299
Dichotomous Expression of TNF Superfamily Ligands on Antigen-Presenting Cells Controls Post-priming Anti-viral CD4(+) T Cell Immunity.
Chang YH, Wang KC, Chu KL, Clouthier DL, Tran AT, Torres Perez MS, Zhou AC, Abdul-Sater AA, Watts TH
Immunity 2017 Nov 21;47(5):943-958.e9
Immunity 2017 Nov 21;47(5):943-958.e9
TRIB3 Promotes APL Progression through Stabilization of the Oncoprotein PML-RARα and Inhibition of p53-Mediated Senescence.
Li K, Wang F, Cao WB, Lv XX, Hua F, Cui B, Yu JJ, Zhang XW, Shang S, Liu SS, Yu JM, Han MZ, Huang B, Zhang TT, Li X, Jiang JD, Hu ZW
Cancer cell 2017 May 8;31(5):697-710.e7
Cancer cell 2017 May 8;31(5):697-710.e7
CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity.
Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, Yang Y, Chien CD, Seif AE, Lei H, Song YK, Khan J, Lee DW, Mackall CL, Gardner RA, Jensen MC, Shern JF, Fry TJ
Nature communications 2016 Jul 27;7:12320
Nature communications 2016 Jul 27;7:12320
Pharmacological Inhibition of the Histone Lysine Demethylase KDM1A Suppresses the Growth of Multiple Acute Myeloid Leukemia Subtypes.
McGrath JP, Williamson KE, Balasubramanian S, Odate S, Arora S, Hatton C, Edwards TM, O'Brien T, Magnuson S, Stokoe D, Daniels DL, Bryant BM, Trojer P
Cancer research 2016 Apr 1;76(7):1975-88
Cancer research 2016 Apr 1;76(7):1975-88
Induction of autophagy is a key component of all-trans-retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacologic modulation.
Orfali N, O'Donovan TR, Nyhan MJ, Britschgi A, Tschan MP, Cahill MR, Mongan NP, Gudas LJ, McKenna SL
Experimental hematology 2015 Sep;43(9):781-93.e2
Experimental hematology 2015 Sep;43(9):781-93.e2
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
Lim S, Kim WJ, Kim YH, Lee S, Koo JH, Lee JA, Yoon H, Kim DH, Park HJ, Kim HM, Lee HG, Yun Kim J, Lee JU, Hun Shin J, Kyun Kim L, Doh J, Kim H, Lee SK, Bothwell ALM, Suh M, Choi JM
Nature communications 2015 Sep 15;6:8244
Nature communications 2015 Sep 15;6:8244
STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma.
Li W, Xiao J, Zhou X, Xu M, Hu C, Xu X, Lu Y, Liu C, Xue S, Nie L, Zhang H, Li Z, Zhang Y, Ji F, Hui L, Tao W, Wei B, Wang H
The Journal of clinical investigation 2015 Nov 2;125(11):4239-54
The Journal of clinical investigation 2015 Nov 2;125(11):4239-54
Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells.
Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, Fusai G, Bertoletti A, Cantrell DA, Kennedy PT, Davies NA, Haniffa M, Maini MK
Nature medicine 2015 Jun;21(6):591-600
Nature medicine 2015 Jun;21(6):591-600
Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis.
Bareja A, Holt JA, Luo G, Chang C, Lin J, Hinken AC, Freudenberg JM, Kraus WE, Evans WJ, Billin AN
PloS one 2014;9(2):e90398
PloS one 2014;9(2):e90398
Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity.
Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, DeKruyff RH, Umetsu DT
Nature medicine 2014 Jan;20(1):54-61
Nature medicine 2014 Jan;20(1):54-61
Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells.
Balan S, Ollion V, Colletti N, Chelbi R, Montanana-Sanchis F, Liu H, Vu Manh TP, Sanchez C, Savoret J, Perrot I, Doffin AC, Fossum E, Bechlian D, Chabannon C, Bogen B, Asselin-Paturel C, Shaw M, Soos T, Caux C, Valladeau-Guilemond J, Dalod M
Journal of immunology (Baltimore, Md. : 1950) 2014 Aug 15;193(4):1622-35
Journal of immunology (Baltimore, Md. : 1950) 2014 Aug 15;193(4):1622-35
Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages.
Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT
Clinical cancer research : an official journal of the American Association for Cancer Research 2013 Jun 15;19(12):3165-75
Clinical cancer research : an official journal of the American Association for Cancer Research 2013 Jun 15;19(12):3165-75
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) preserves gut barrier function by blocking neutrophil-endothelial cell adhesion after hemorrhagic shock and resuscitation in mice.
Zhang HY, James I, Chen CL, Besner GE
Surgery 2012 Apr;151(4):594-605
Surgery 2012 Apr;151(4):594-605
Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties.
Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF
Neuro-oncology 2010 Apr;12(4):351-65
Neuro-oncology 2010 Apr;12(4):351-65
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
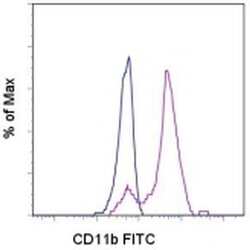
- Experimental details
- Staining of normal human peripheral blood cells with Mouse IgG1 K Isotype Control FITC (Product # 11-4714-42) (blue histogram) or Anti-Human CD11b FITC (purple histogram).
- Conjugate
- Green dye
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
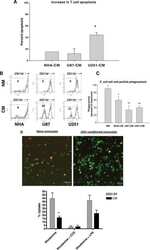
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
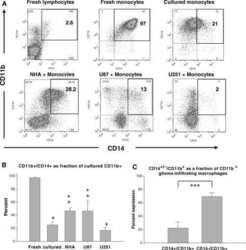
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
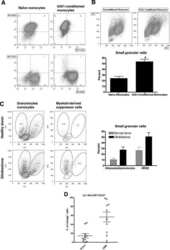
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
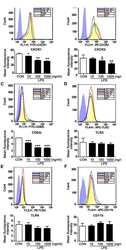
- Fig 5 Knockdown of HDAC2 in HL-60 cells caused differentiation inhibition when PAX5 was also depleted. (A) GO terms of atypically active genes regulated by HDAC2 were analyzed in gene ontology consortium. X-axis represents the adjusted P-value transformed by -log10, and Y-axis denotes the enriched GO terms. (B) HL-60 cell differentiation was measured in control and sh HDAC2 3'UTR and/or sh PAX5 3'UTR HL-60 cells by CD11b staining. Cells were stained with CD11b-PE for 1 hr, and analyzed by FACS.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
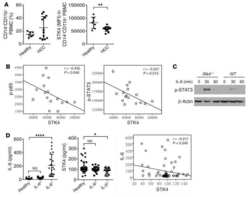
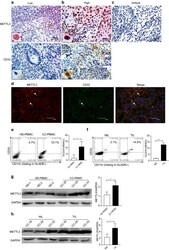
- Fig. 1 METTL3 expression and CD33 + MDSC distribution in patients with CC. a , b The immunohistochemical staining for METL3 and CD33 CC specimens (x 400). c The isotype antibody IgG was included (x 400). d Immunofluorescence staining for METTL3 (red) and CD33 + (green) in CC specimens; the white arrows point to the METTL3 + and CD33 + cells. The images were taken by fluorescence microscope. HLA-DR - CD33 + CD11b + cells were gated by a FACS gating strategy and were defined as MDSCs in this study. e , f Representative density plots showed the MDSC population in the peripheral blood of healthy donors (HD) or CC patients, as well as in the immune cells from tumour tissues (TIL) or tumour-adjacent tissues (NIL). A statistical graph is included for the comparison between the indicated groups. (G-H) Representative immunoblotting shows the expression of METTL3 in the peripheral blood, TILs and NILs. A statistical graph is included for the comparison between the indicated groups. The experiments in e , f were performed at least three times, and the data were plotted as the mean +- SEM. Statistics were conducted with an unpaired Student''s t test, * P < 0.05, and *** P < 0.001 vs. the corresponding control
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
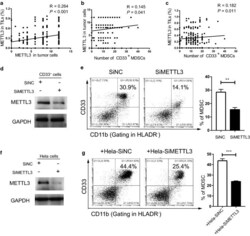
- Fig. 2 Tumour METTL3 level was positively related to intratumoural CD33 + MDSCs in vivo and CC-derived MDSCs in vitro. a The association between METTL3 expression in tumour cells and the expression of METTL3 in TILs (R = 0.264, P < 0.001). b The association between METTL3 expression in tumour cells and intratumoural CD33 + MDSC number (R = 0.145, P = 0.041). c The association between METTL3 expression in TILs and intratumoural CD33 + MDSC number (R = 0.182, P = 0.011). CD33 + cells were isolated from PBMCs of healthy donors with human anti-CD33 beads, and the METTL3 levels in CD33 + cells or HeLa cells were knocked down by siMETTL3. d Immunoblotting showed the METTL3 expression in CD33 + cells with or without METTL3 knockdown. e HLA-DR - CD33 + CD11b + MDSC induction from CD33 + cells in the presence of siMETTL3 or siControl (SiNC). A statistical graph is included for the comparison between the indicated groups. f Immunoblotting showed METTL3 expression in HeLa cells with or without METTL3 knockdown. g Tumour-associated HLA-DR - CD33 + CD11b + MDSC induction from CD33 + cells in coculture with Hela-siMETTL3 or Hela-siControl cells in a Transwell System for 48 h. A statistical graphs is included for the comparison between the indicated groups. Representative flow cytometry density plots (left) and statistical bar chart (right). The statistical analysis was performed using Spearman''s correlation and linear regression. R, Spearman''s correlation, is the correlation coefficient.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
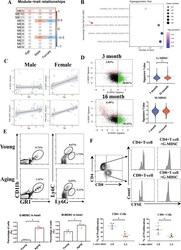
- Fig. 1 Levels of MDSCs in the heart are positively associated with age. A WGCNA of the coexpressed genes from 135 human whole blood transcriptomes from the GEO database (GSE123698). ME0 was related to age (correlation coefficient = 0.15) but not to sex (correlation coefficient = 0.043). Red indicates a positive correlation, and blue indicates a negative correlation. B Hypergeometric test of the genes in ME0 based on the immunologic gene set from GSE123698. The size of the dot represents the number of genes. A darker color indicates a lower correlation. C The bubble plots show the correlation between the G-MDSC/M-MDSC signature and age in males and females based on GSE123698. D The ratio (left) and signature value (right) of the G-MDSCs and M-MDSCs in 3-month-old and 16-month-old mice determined by scRNA-Seq analysis (GSE145477). E Representative flow cytometric profile showing the quantitative analysis of Cd11b + Gr1 + Ly6G+ cells and Cd11b + Gr1 + Ly6C+ cells in the hearts of young and aging mice; n = 5 per group. F CFSE-labeled CD4 + and CD8 + T cells and G-MDSCs separated from hearts were cocultured at a ratio of 2:1 for 24 h. Representative images and quantitative analysis of the proliferation of CD4 + or CD8 + T cells analyzed by flow cytometry; n = 6 per group. The data are presented as the means +- SDs. Differences were determined by Student's t test. * P < 0.05.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
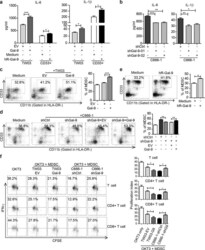
- Fig. 2 The effect of intra- and extra-cellular Gal-9 on MDSC differentiation and expansion in vitro. ELISA assay of IL-6 and IL-1beta concentrations in the conditioned media of TW03-EV and TW03-Gal-9 cells or CD33 + cells ( a ) or Gal-9 knockdown (shGal9-01 and shGal9-02) and control C666-1 cells ( b ) with or without addition of human recombinant Gal-9 (hR-Gal-9). c Differentiation of CD33 + cells isolated from healthy PBMCs investigated after treatment with control medium, co-cultivation with TW03-EV or TW03-Gal-9. Representative flow cytometry plots ( left ) and histogram ( right ) of MDSC differentiation assays showing the percentage of CD33 + CD11b + cells in the HLA-DR - gate arising from CD33 + cells. d Similar experiment based on co-cultivation with C666-1 cells using four experimental conditions: C666-1-shCtrl cells (shCtrl), unmodified C666-1-shGal9-01 (shGal-9), C666-1-shGal9-01 co-transfected with a control lentiviral vector (shGal9 + EV) or a Gal-9 lentiviral vector (ShGal-9+Gal-9). e Representative flow cytometry plots ( left ) and histogram ( right ) of MDSC differentiation assays showing the percentage of CD33 + CD11b + cells in the HLA-DR - gate arising from CD33 + cells with or without addition of human recombinant Gal-9 (hR-Gal-9). f Representative flow cytometry plots ( left ) and histogram ( right ) of the IFN-gamma-positive, proliferating T cells (CSFE assay) to test the immune-suppressive activity of MDSC cells induced by TW03-EV and TW03-Gal-9 or C666-
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
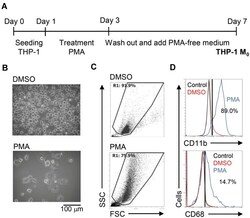
- Figure 1 Phorbol 12-myristate 13-acetate (PMA) treatment triggers macrophage differentiation in human monocytic THP-1 cells. ( A ) Experimental flowchart of the PMA stimulation performed in this study. ( B ) Cell morphology evaluation showed cell growth in PMA-treated THP-1 cells. ( C ) Flow cytometric dot-plot, plotting forward-scattered (FSC) versus side-scattered (SSC) from a population of THP-1 cells, showing the cell size and complexity. ( D ) Immunostaining followed by flow cytometric histogram analysis showed the expression of CD11b and CD68. Treatment of DMSO was used as control. For all images and flow cytometric analysis, representative staining data of isotype control, DMSO, and PMA were selectively obtained from three individual experiments. For the flow cytometric analysis, the percentage of positive cells in PMA treatment is shown.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
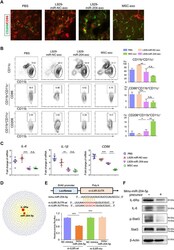
- Fig. 7. miR-204 was the candidate mediator of MSC-exo for macrophage reprogramming from M1 to M2 by targeting IL-6R signaling. ( A ) L929-miR-204-exo and MSC-exo treatments suppressed the CD86 + M1 macrophages and promoted CD206 + M2 ones in the BAC-induced corneal whole-mount staining. Scale bar, 50 mum. ( B ) Flow cytometry showed that, in the BAC-induced dry eye corneas, the percentage of CD11b + CD11c - macrophages decreased after L929-miR-204-exo or MSC-exo treatment compared with L929-miR-NC-exo controls, with more prominent reduction in the MSC-exo group. In addition, both treatments inhibited the percentage of CD11b + CD11c - CD86 + M1 macrophages to a similar extent. n = 12 eyes (four corneas mixed in one sample), one-way ANOVA and Tukey's post hoc test. ( C ) mRNA expressions of IL-6, IL-1beta, and CD86 were decreased after L929-miR-204-exo and MSC-exo treatments in comparison to the L929-miR-NC-exo group. n = 6 eyes, one-way ANOVA and Tukey's post hoc test. ( D ) Predicted regulatory networks of genes targeted by miR-204. ( E ) Luciferase reporter assay demonstrating direct interaction of miR-204-5p with 3'UTR of the Il-6r gene. One-way ANOVA and Tukey's post hoc test. n = 3. * P < 0.05, ** P < 0.01, and *** P < 0.001. ( F ) Western blot analysis showing the suppression of IL-6Ralpha, IL-6, and p-Stat3 by mmu-miR-204-5p precursor.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6 Effects of Lnc-S100B-2 and MLLT10 on the tumor microenvironment. (a) The expressions of CD3 were detected by IF assay. (b) The percent of CD3 (-) CD16 (+) cells was detected by flow cytometry. (c) The percent of CD11b (+) cells was analyzed by flow cytometry. (d, e) The expression E-cadherin and vimentin was analyzed by IF assay. (f-l) The expressions of E-cadherin, N-cadherin, vimentin, beta -catenin, snail, and slug were detected by qRT-PCR and Western blot. * P < 0.05 versus NC group, # P < 0.05 versus sh-MLLT10 group, & P < 0.05 versus sh-Lnc-S100B-2 group, and one-way ANOVA.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Cell proliferation and differentiation of THP-1 after treatment with 5 muM of 5-AZA and lentivirus transfection. (A) The proliferative capacity of THP-1 after treatment with 5 muM of 5-AZA and lentivirus transfection by MTT assay (* p < 0.05). (B) CD11b and CD14 expressions of THP-1 after treatment with 5 muM of 5-AZA and lentivirus transfection by flow cytometry (* p < 0.05).
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
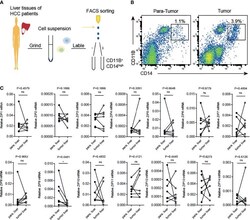
- Experimental details
- Transcription of Zip9 is downregulated in HCC patients-derived TAMs. (A) FACS protocol was used for isolating HCC patients-derived TAMs. (B) Human liver cancer tissues and paracarcinoma liver tissues were digested, stained for CD14, CD11B and then analyzed by flow cytometry. (C) The mRNA levels of ZIPs in TAMs isolated from human liver cancer tissues and paracarcinoma liver tissues were detected by RT-PCR (n = 7). The black points represent the individual paracarcinoma liver tissue, and the black circles represent the liver cancer tissue. The expressed values were calculated by the 2 -DeltaCT method. *P < 0.05, ns, not significant. The representative data from at least three independent experiments are shown. Data were determined with 2-tailed unpaired Student's t test between 2 groups.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
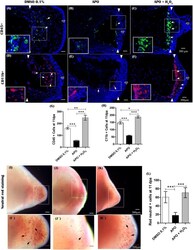
- Experimental details
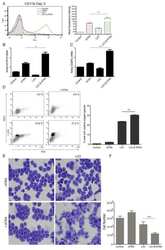
- FIGURE 9 The production of ROS generated post-amputation of the limb is necessary for the recruitment and phagocytic activity of inflammatory cells. (A-C) , Representative immunofluorescence images against the leukocyte pan marker CD45 at 11 dpa. Boxes in white dotted lines are shown at higher magnification in solid line boxes for each image. (A) CD45 + cells are predominantly located in the blastema region adjacent to the amputation plane. (B) A reduced number of CD45 + cells are observed near the amputation plane. (C) a notable increase of CD45 + cells are observed in the region of the blastema and the amputation plane. (D-F) , Representative immunofluorescence images against CD11b. A higher presence of CD11b + cells can be seen in the control group compared with the apocynin-treated group. Animals exposed to rescue treatment show a prominent accumulation of CD11b + cells in the blastema and amputation plane. (G,H) , quantification of CD45 and CD11b + positive cells, respectively. (I-K) , vital staining with neutral red at 11 dpa. Representative images of control animals in 0.1% DMSO, exposed to apocynin inhibitor (APO) and rescue assays. Boxes in dotted white lines are shown at higher magnification in (I',j',K') for each experimental group. (L) , quantification of cells positive for neutral red staining. Data are expressed as mean +- SEM. One-way ANOVA followed by Tukey's post hoc test was performed for comparisons between groups treated with apocynin, exogenous H 2 O 2 an
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
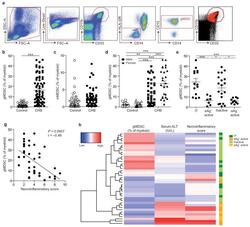
- Experimental details
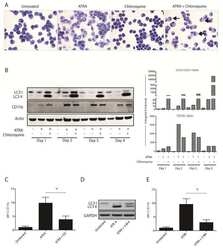
- Figure 1 gMDSC expand in subjects replicating HBV in the absence of immunopathology a ) Sequential gating strategy for gMDSC identification (CD11b high CD33 + HLA-DR - CD14 - CD15 + ) using 11-color flow cytometry from freshly isolated PBMC (doublet discrimination not shown). gMDSC population (superimposed in red) was calculated as a percentage of myeloid cells (CD11b high CD33 + ). Cumulative dot plots showing circulating b ) gMDSC and c ) mMDSC frequencies (n=44, healthy controls; n=84, CHB). d ) gMDSC frequencies analyzed by gender. e ) Summary plot of frequencies classified by disease phase using a subset of the cohort with clearly defined disease phases: 14 ""immunotolerants"" (HBeAg + , HBV DNA >10 7 IU/ml, ALT 5x10 5 IU/ml, ALT >60 IU/L), 21 ""inactive disease"" (HBeAg - , HBV DNA 60 IU/L). f ) gMDSC frequencies according to hepatic necroinflammatory score (n=42, CHB). g ) Unsupervised hierarchical clustering using Euclidean distance; dendrogram displaying similarity between clusters. Clinically assigned disease phase, shown adjacent to plot; immunotolerant: dark green, eAg + active disease: dark yellow, inactive disease: pale green, eAg - active disease: pale yellow (not used for analysis). Increasing color intensity (blue-red) corresponds to increasing gMDSC frequency, ALT (IU/L) or necroinflammatory score (n=42, CHB; maximum
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
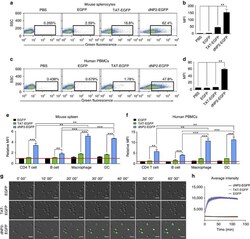
- Experimental details
- Figure 2 Protein delivery efficiency of dNP2 in primary mouse and human immune cells. ( a , b ) Mouse primary splenocytes were isolated from 6-week-old female C57BL/6 mice and the cells were incubated with 5 muM EGFP, TAT- and dNP2-EGFP for 2 h. Intracellular fluorescence was analysed by flow cytometry and the data are represented as dot plots or mean fluorescence intensity (MFI) of the cells. ( c , d ) Human PBMCs were isolated from healthy donor blood and the cells were incubated with 5 muM EGFP, TAT-, dNP2-EGFP for 2 h. The data were analysed as described above. ( e ) Total splenocytes were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated using markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11c lo CD11b hi F480 + ) and DCs (CD11c hi MHCII hi ). The EGFP signal in each cell population was then analysed by flow cytometric analysis. The relative MFI value was normalization to PBS treated cells. The red line indicates relative MFI of PBS-treated cells. ( f ) Total PBMCs were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated with markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11b + ) and DCs (CD11c + ) and the data were then analysed as described above. ( g ) Time-lapse images of mouse CD4 T cells incubated with 1 muM EGFP, TAT- and dNP2-EGFP were acquired for 2 h (Scale bar, 15 mum) and ( h ) the average fluorescence intensities of 10 cells from each sample were calculate
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
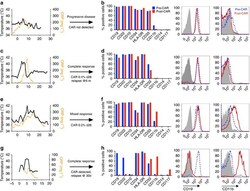
- Figure 1 Phenotypic alterations in clinical ALL samples following CD19 CAR. ( a , c , e , g ) Fever curves, CRP values and clinical response to CD19 CAR treatment. ( b , d , f , h ) Bar graph demonstrating percent of cells expressing cell surface markers by flow cytometry in the bone marrow, gated on leukaemic blasts, with representative flow cytometry histograms of CD19 and CD11b on the right (grey, control; blue, pre-CAR; red, post CAR). ( a , b ) Pre-CAR sample and day +30 post-CAR sample from a patient who did not experience CRS following CD19 CAR. ( c , d ) Pre-CAR and +180 days post-CAR samples from a patient with normal karyotype multiple relapsed ALL, who had a severe CRS followed by an MRD-negative complete response with CD19 CAR, with no CAR + cells persisting beyond 60 days. ( e , f ) Pre-CAR and day +30 post-CAR samples from a patient with normal karyotype ALL who experienced a mild CRS and CAR expansion, but had persistent disease. ( g , h ) Pre-CAR and post-CAR samples from an infant with MLL-rearranged ALL, treated with CD19-41BB-zeta CAR, who relapsed with myeloid blasts.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
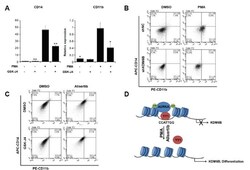
- Fig. 5 KDM6B promotes the differentiation of THP-1 cells (A) THP-1 cells were treated with 100 ng/ml PMA, 2 muM GSK-J4, or DMSO for 48 h. CD14 and CD11b expression levels were confirmed using qRT-PCR and normalized to GAPDH . Results are shown as mean +- SEM, n = 3; *p < 0.05, **p < 0.01. (B) We treated negative control (shNC)- and shKDM6B-transfected THP-1 cells with 100 ng/ml PMA for 48 h. The cells were stained with PE-CD11b and APC-CD14 antibodies. The percentage of cells in each quadrant is indicated in the figure. (C) We treated THP-1 cells with 2 muM GSK-J4 or 0.3 muM alisertib for 48 h. The cells were stained with PE-CD11b and APC-CD14 antibodies. The percentage of cells in each quadrant is indicated in the figure. (D) A model of AURKA regulating KDM6B expression in PMA-mediated THP-1 differentiation.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
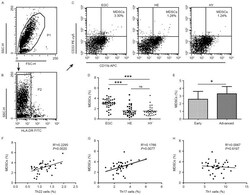
- Figure 2. Flow cytometric analysis of MDSCs in peripheral whole blood from EGC (n=39), HE (n=32) and HY (n=31). Gating routine in (A) P1 and (B) P2 successively for MDSCs (HLA-DR - CD33 + CD11b + ) subsets and (C) representative results of flow cytometric analyses for MDSCs in the three groups of subjects. The number of cells in EGC, HE and HY in P2 were 8,394, 8,004 and 8,224, respectively. (D) The proportion of MDSCs in the three groups of subjects. (E) The proportion of MDSCs in peripheral whole blood derived from patients with early (n=13) or advanced (n=26) gastric cancer. The association between the proportion of MDSCs and (F) Th22, (G) Th17 and (H) Th1 cells in peripheral whole blood of elderly patients with cancer. *P
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
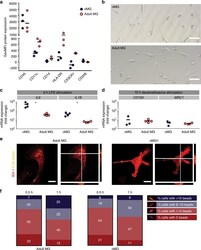
- Fig. 4 oMG expressed microglia-characteristic cell surface markers and showed similar functional immune and phagocytic properties as adult MG. a Flow cytometric analyses of the expression pattern of microglial extracellular markers on CD11b+-gated oMG (oMG 1, 3, and 5) compared to adult MG derived from three separate brain regions from adult MG1.1. (eight organoids were pooled per donor (oMG 1, 3, and 5) after 52 days in culture). b Morphology of magnetic automated cell sorted CD11b+ oMG 1 and adult MG in bright field microscope after 1 week in culture. Scale bar 40 mum. c mRNA expression, determined by qRT-PCR, of pro-inflammatory cytokines IL6 and IL1B after 6 h stimulation with LPS was significantly higher in oMG compared to adult MG (Mann-Whitney test IL6 and IL1B: U = 0, n = 4, p = 0.03). LPS-stimulated response relative to control condition without LPS. ( n = 4 experiments, eight organoids pooled per experiment; adult MG1.1) (* p < 0.05). d Anti-inflammatory response of oMG and adult MG was compared by qRT-PCR for expression of anti-inflammatory genes CD163 and MRC1 upon 72 h stimulation with dexamethasone. Dexamethasone-stimulated response relative to control condition without dexamethasone. (oMG, n = 3 separate experiments in which oMG were isolated from > 4 pooled cerebral organoids from iPSC 1 per experiment; adult MG, n = 4). e Phagocytosis capacity was tested oMG 1 and adult MG by performing a phagocytosis assay with iC3b-coated green-yellow
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
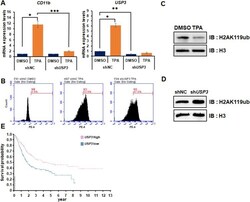
- Experimental details
- Figure 4. Depletion of USP3 inhibited TPA-mediated HL-60 cell differentiation via regulating H2A119ub. (A) HL-60 cells are analyzed by RT-qPCR to examine the mRNA expression levels of CD11b and USP3 . Cells are treated with DMSO or TPA (32 nM) for 48 h. Results are represented as mean +- SEM; n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001. (B) Differentiation of HL-60 cells (CD11b positive) are measured by FACS analysis performed in shNC DMSO, shNC TPA, and shUSP3 TPA. Cells are stained with CD11b-PE for 30 min and analyzed by FACS. (C,D) HL-60 cells are treated with DMSO or TPA for 48 h. Purified histones are resolved by SDS-PAGE and immunoblotted with anti-H3 or anti-H2AK119ub antibodies. (E) USP3 expression levels are showed in comparison with various types of leukemia via the Oncomine database. (F) The probability of survival in urothelial cancer patient is showed in comparison with the level of USP3 expression.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
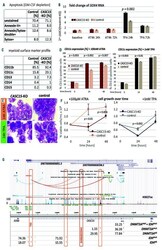
- Experimental details
- Figure 1. CASC15 -KO promotes the differentiation of acute myeloid leukemia cells. (A) Apoptosis in CASC15 -KO and empty vector-transduced (control) OCI-AML5 cell lines after 24 h of depletion of granulocyte-macrophage colony-stimulating factor (annexin-FITC/Sytox blue flow cytometry). (B) Expression of SOX4 during in vitro differentiation of CASC15 -KO and control OCI-AML5 cell lines. All cells were treated with 0.1 mM all- trans retinoic acid (ATRA) and 1 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) over 72 h in three independent experiments. Total RNA was extracted before, after 24 h and after 72 h of treatment, DNase-digested and transcribed to cDNA. A quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR green chemistry with subsequent melting curve analysis in technical triplicates. The 2-ddCt was calculated relative to the pre-determined housekeeping gene encoding succinate dehydrogenase complex subunit C ( SDHC ). (C) Baseline expression of the monocyte/macrophage markers CD11b (integrin subunit alpha M, ITGAM), CD11c (integrin subunit alpha X, ITGAX), and CD14, the granulocyte marker CD15 (fucosyltransferase 4, FUT4), and the general myeloid marker CD13 (aminopeptidase N, APN) in CASC15 -KO and control cells. The percentages of positive cells, quantified by flow cytometry after 72 h, are shown. (D-F) Growth rate and CD11c myeloid cell surface marker expression of CASC15 and control cell lines during drug-induced in vitro differentiation
- Conjugate
- Green dye
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry